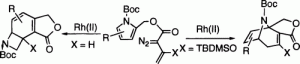Authors: Huw M. L. Davies , Julius J. Matasi , and Gulzar Ahmed
J. Org. Chem.,
1996, 61 (7), 2305–2313
The rhodium(II)-catalyzed intramolecular reaction between vinyldiazomethanes and pyrroles leads to a novel synthesis of fused tropanes. The reaction occurs by a stepwise 3 + 4 annulation mechanism between a rhodium-stabilized vinylcarbenoid intermediate and the pyrrole rather than by the typical tandem cyclopropanation/Cope rearrangement sequence. The outcome of the reaction is very sensitive to the vinylcarbenoid structure. In particular, the presence of a 2-siloxy substituent on the vinylcarbenoid strongly favors the formation of fused tropanes. In the absence of such functionality, the formation of fused 7-azabicyclo[4.2.0]octadienes becomes the dominant reaction pathway.