Authors: Huw M.L. Davies, Darrin W. Hopper, Tore Hansen, Quixu Liu, Steven R. Childers
Bioorganic & Medicinal Chemistry Letters
2004, 14, 7, 1799-1802
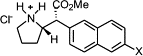
The rhodium(II)-catalyzed intermolecular C–H insertion of methyl aryldiazoacetates with either N-Boc-piperidine or N-Boc-pyrrolidine followed by deprotection with trifluoroacetic acid is a very direct method for the synthesis of methylphenidate analogues. By using either dirhodium tetraacetate or dirhodium tetraprolinate derivatives as catalyst, either the racemic or enantioenriched methylphenidate analogues can be prepared. The binding affinities of the methylphenidate analogues to both the dopamine and the serotonin transporters are described. The most notable compounds are the erythro-(2-naphthyl) analogues which display high binding affinity and selectivity for the serotonin transporter.