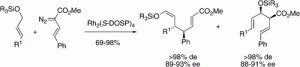Authors: Huw M. L. Davies and Rohan E. J. Beckwith
J. Org. Chem.,
2004, 69 (26), 9241–9247
Tetrakis(N-[4-dodecylbenzenesulfonyl]-(l)-prolinate) dirhodium [Rh2(S-DOSP)4]-catalyzed decomposition of vinyldiazoacetates in the presence of allyl silyl ethers results in the formation of the direct C−H insertion product and the product derived from a combined C−H activation/siloxy-Cope rearrangement. Both products are formed with very high diastereoselectivity (>94% de) and high enantioselectvity (78−93% ee). Under thermal or microwave conditions, the direct C−H insertion product undergoes a siloxy-Cope rearrangement in a stereoselective manner.