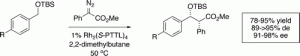Authors: Huw M. L. Davies , Simon J. Hedley , and Brooks R. Bohall
J. Org. Chem.,
2005, 70 (26), 10737–10742
C−H functionalization of benzyl silyl ethers by means of rhodium-catalyzed insertions of aryldiazoacetates can be achieved in a highly diastereoselective and enantioselective manner by judicious choice of chiral catalyst or auxiliary. The dirhodium tetraprolinates such as Rh2((S)-DOSP)4 have been widely successful as chiral catalysts in the C−H functionalization chemistry of aryldiazoacetates, but give poor enantioselectivity in the reactions of aryldiazoacetates with benzyl silyl ether derivatives. The use of (S)-lactate as a chiral auxiliary resulted in C−H functionalization with moderately high diastereoselectivity (79−88% de) and enantioselectivity (68−85% ee). The best results (91−95% de, 95−98% ee), however, were achieved using Hashimoto’s Rh2((S)-PTTL)4 catalyst.