Authors: Huw M.L. Davies, Jaemoon Yang, Joachim Nikolai
J. Organomett. Chem.,
2005, 690, 24-25, 6111-6124
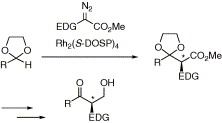
The dirhodium tetraprolinate, Rh2(S-DOSP)4 is an efficient catalyst in an enantioselective C–H activation protocol. Rh2(S-DOSP)4 catalyzed decomposition of aryldiazoacetates or vinyldiazoacetates results in the formation of transient rhodium carbenoid intermediates. These intermediates are capable of selectively inserting into the C–H bond of acetals. The resulting products are protected β-keto esters, and so the C–H activation protocol can be considered as strategically equivalent to the Claisen condensation.