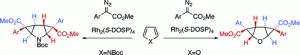Authors: Simon J. Hedley , Dominic L. Ventura , Paulina M. Dominiak , Cara L. Nygren , and Huw M. L. Davies
J. Org. Chem.,
2006, 71 (14), 5349–5356
Rhodium-catalyzed decomposition of aryldiazoacetates in the presence of pyrroles or furans results in mono- or biscyclopropanation of the heterocycle, but with opposite enantioinduction. In the absence of sterically encumbering groups, the cyclopropanation of furan occurs with initial bond formation at the 2-position. If this pathway is sterically blocked, cyclopropanation can occur with initial bond formation at the 3-position of the furan ring; in this case, the cyclopropanation reaction takes place on the opposite face of the heterocycle, and the opposite enantioinduction is observed. Upon extension of this methodology to benzofurans, a highly enantioselective monocyclopropanation reaction occurs to furnish a product derived from initial bond formation at the 2-position of the benzofuran. When this reaction pathway is inhibited by sterically encumbering substituents on the benzofuran, no cyclopropanation of the furan ring is observed, and instead, double cyclopropanation of the benzene ring occurs. Double cyclopropanation of the benzene ring was also observed in reactions with indoles.