Authors: Huw M.L. Davies, Xing Dai
Tetrahedron,
2006, 62, 45, 10477-10484
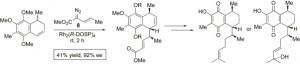
The enantioselective total syntheses of the assigned structure of (+)-elisabethadione (3) and the (+)-p-benzoquinone natural product 4 is described. The stereocontrolled formation of the three key stereocenters in the natural products is achieved in a single step through the combined C–H activation/Cope rearrangement, a C–H functionalization process catalyzed by the dirhodium tetraprolinate, Rh2(R-DOSP)4 (DOSP=(N-dodecylbenzenesulfonyl)prolinate).