Authors: Yulia Sevryugina, Beth Weaver, Jørn Hansen, Janelle Thompson, Huw M. L. Davies and Marina A. Petrukhin
Organometallics,
2008, 27 (8), 1750–1757
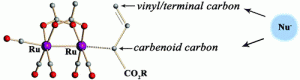
Highly electrophilic ruthenium(I) mixed carbonyl carboxylate complexes exhibit catalytic activity in the cyclopropanation of styrene with methyl phenyldiazoacetate. A particular advantage of these catalysts is their propensity to enhance vinylogous reactivity in the reactions of vinyldiazoacetates. The catalytic study was conducted on four known ruthenium(I) mixed carbonyl carboxylate complexes and four new complexes, namely, [Ru2(O2C(2,3,4-F)3C6H2)2(CO)5], [Ru2(O2C(2,4,6-F)3C6H2)2(CO)5], [Ru2(O2CC6F5)2(CO)5], and [Ru2(O2C(3,5-CF3)2C6H3)2(CO)4]. All complexes have been prepared by a combination of solvent-free techniques: melt reactions of ruthenium carbonyl with a benzoic acid followed by gas-phase sublimation−deposition of the products under reduced pressure. X-ray crystallographic characterization revealed a tetranuclear “dimer of dimers” type of structure for the [Ru2(O2CR)2(CO)5] complexes and a polymeric chain for [Ru2(O2C(3,5-CF3)2C6H3)2(CO)4]. Both motifs are built on diruthenium(I,I) units linked in the solid state by axial Ru···O interactions. The solution behavior of the polynuclear ruthenium(I) complexes in solvents of varying coordination ability has been investigated to show a breakage of weak Ru···O contacts, resulting in the formation of one- and two-end open dimetal units.