Authors: Justin R. Denton and Huw M. L. Davies
Org. Lett.,
2009, 11 (4), 787–790
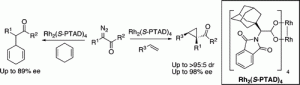
The reaction of a variety of α-aryl-α-diazo ketones with activated olefins, catalyzed by the adamantyl glycine-derived dirhodium complex Rh2(S-PTAD)4, generates cyclopropyl ketones with high diastereoselectivity (up to >95:5 dr) and enantioselectivity (up to 98% ee). Intermolecular C−H functionalization of 1,4-cyclohexadiene by means of carbenoid-induced C−H insertion was also possible with this type of carbenoid.