Authors: Brett D. Schwartz, Justin R. Denton, Yajing Lian, Huw M. L. Davies and Craig M. Williams
J. Am. Chem. Soc.,
2009, 131 (23), 8329–8332
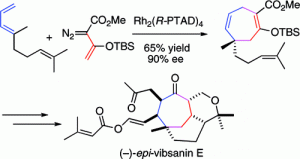
The total synthesis of (−)-5-epi-vibsanin E (2) has been achieved in 18 steps. The synthesis combines the rhodium-catalyzed [4 + 3] cycloaddition between a vinylcarbenoid and a diene to rapidly generate the tricyclic core with an effective end game strategy to introduce the remaining side-chains. The [4 + 3] cycloaddition occurs by a cyclopropanation to form a divinylcyclopropane followed by a Cope rearrangement to form a cycloheptadiene. The quaternary stereogenic center generated in the process can be obtained with high asymmetric induction when the reaction is catalyzed by the chiral dirhodium complex, Rh2(S-PTAD)4.