Authors: John F. Briones, Jørn Hansen, Kenneth I. Hardcastle, Jochen Autschbach, and Huw M. L. Davies
J. Am. Chem. Soc.,
2010, 132 (48), 17211–17215
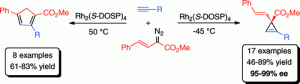
Dirhodium tetrakis((S)-N-(dodecylbenzenesulfonyl)prolinate) (Rh2(S-DOSP)4) is an effective catalyst for highly enantioselective cyclopropenation reactions between terminal alkynes and arylvinyldiazoacetates. The resulting vinylcyclopropenes can undergo rhodium-catalyzed regioselective rearrangement to cyclopentadienes. Computational studies indicate that the high enantioselectivity of the process is governed by the specific orientation of the alkyne during its approach to the carbenoid through a relatively late transition state. The specific orientation occurs due to the presence of a hydrogen bonding interaction between the alkyne hydrogen and a carboxylate ligand on the dirhodium catalyst.