Authors: John F. Briones and Huw M. L. Davies
J. Am. Chem. Soc.,
2013, 135 (36), 13314–13317
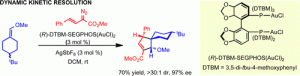
The reaction of vinyldiazoacetates with enol ethers catalyzed by the binuclear gold complex (R)-DTBMSegphos(AuCl)2 activated by silver hexafluoroantimonate results in a highly enantioselective [3 + 2] cycloaddition. The [3 + 2] cycloaddition proceeds with dynamic kinetic resolution when the enol ether is a 4-substituted 1-(methoxymethylene)cyclohexane. The reaction is initiated by nucleophilic attack of the vinyl ethers at the vinylogous position of the gold vinylcarbene intermediate.
Read More