A doctor tells a patient that there are two drugs that are capable of treating his chronic pain: drug ‘A’ and drug ‘B’. Both drugs will be equally effective at treating the pain, but drug ‘A’ is highly addictive while drug ‘B’ is hardly so. The choice seems like a no brainer before even mentioning that drug ‘B’ is cheaper, faster acting, and has fewer negative side effects than drug ‘A’. This is precisely the difference between a narcotic painkiller and marijuana. Yet, to this day, Marijuana wrongfully remains a schedule 1 drug as classified by the United States Government. Under the Controlled Substance Act of the USDA, schedule one drugs are listed as those with “no currently accepted medical use and a high potential for abuse,” (DEA). Although there is some evidence that may support the government’s reasoning, I will argue that it is mostly based on situational studies that fail to keep in mind a variety of variables as well as the benefits of marijuana use in specific medical circumstances given all available treatment options.
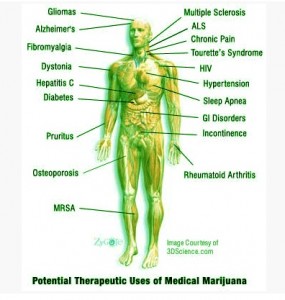 First of all, despite claims that marijuana has no medical uses, there is plenty of evidence that the various psychoactive and non-psychoactive compounds of cannabis could be used to treat multiple symptoms in various illnesses and may even be the only effective form of treatment for some patients. Specifically, cannabis has been shown to have an extremely wide range of treatment possibilities including: halting muscle spasms, alleviating tremors, lowering anxiety, inducing hunger, reducing pain, and even preventing psychosis (Lamorine, Iseger and Bossong). This is due to the multiple neurological effects caused by a combination of the psychoactive THC and non-psychoactive cannabidiols which each have various treatment potentials. Therefore, cannabis may be an effective form of treatment for some patients who would otherwise be at risk for harmful drug interactions from taking multiple prescription drugs to treat many symptoms. For example, although cannabis is primarily perscribed to multiple sclerosis patients for its pain relieving effects, it may also reduce sleep problems and help prevent the wasting syndrome caused by lack of appetite (Rog et. al.). Also, marijuana used to alleviate some of the negative side effects of chemotherapy and cancer such as nausea and tumor growth itself could help to reduce the effects of the anxiety and depression indirectly caused by the cancer (McAllister et.al.). In both of these scenarios, a combination of both THC and cannabidiols is responsible for treating such a wide variety of symptoms. Most importantly, however, cannabis remains the only effective treatment for detrimental illnesses such as glaucoma as well as movement and seizure disorders. In particular, THC is the only known form of treatment for glaucoma, and cannbidiol may be one of the few available treatments for adolescents with seizure disorders like Dravet’s syndrome or Lennox-Gestaut syndrome. (Lamorine, Porter and Jacobson). In addition, recent studies have shown that cannabidiols have antipsychotic effects as well and may be an appropriate treatment for schizophrenia (Isseger and Bossong). It is well known that there is no effective treatment for schizophrenia, so cannabidiols could give schizophrenic patients their first chance to function in society. As you can see, arguments that there are no medical uses for marijuana simply don’t stand up to the available evidence.
First of all, despite claims that marijuana has no medical uses, there is plenty of evidence that the various psychoactive and non-psychoactive compounds of cannabis could be used to treat multiple symptoms in various illnesses and may even be the only effective form of treatment for some patients. Specifically, cannabis has been shown to have an extremely wide range of treatment possibilities including: halting muscle spasms, alleviating tremors, lowering anxiety, inducing hunger, reducing pain, and even preventing psychosis (Lamorine, Iseger and Bossong). This is due to the multiple neurological effects caused by a combination of the psychoactive THC and non-psychoactive cannabidiols which each have various treatment potentials. Therefore, cannabis may be an effective form of treatment for some patients who would otherwise be at risk for harmful drug interactions from taking multiple prescription drugs to treat many symptoms. For example, although cannabis is primarily perscribed to multiple sclerosis patients for its pain relieving effects, it may also reduce sleep problems and help prevent the wasting syndrome caused by lack of appetite (Rog et. al.). Also, marijuana used to alleviate some of the negative side effects of chemotherapy and cancer such as nausea and tumor growth itself could help to reduce the effects of the anxiety and depression indirectly caused by the cancer (McAllister et.al.). In both of these scenarios, a combination of both THC and cannabidiols is responsible for treating such a wide variety of symptoms. Most importantly, however, cannabis remains the only effective treatment for detrimental illnesses such as glaucoma as well as movement and seizure disorders. In particular, THC is the only known form of treatment for glaucoma, and cannbidiol may be one of the few available treatments for adolescents with seizure disorders like Dravet’s syndrome or Lennox-Gestaut syndrome. (Lamorine, Porter and Jacobson). In addition, recent studies have shown that cannabidiols have antipsychotic effects as well and may be an appropriate treatment for schizophrenia (Isseger and Bossong). It is well known that there is no effective treatment for schizophrenia, so cannabidiols could give schizophrenic patients their first chance to function in society. As you can see, arguments that there are no medical uses for marijuana simply don’t stand up to the available evidence.
One slightly more valid argument against the use of medical marijuana, that the drug has a ‘high potential for abuse’ and may endanger the patients physical and psychological well being, is based on misconceptions about the drug’s addictive properties and relies on evidence that fails to account for necessary compounding variables. There have been some studies in recent years providing evidence that squirrel monkeys will repeatedly self-administer cannabis intra-ventricularly (Justinova et. al). This makes sense because separate studies have revealed that there are some of the CB1 receptors that are responsible for the psychoactive effects of cannabis, on GABA-ergic interneurons in the VTA and nucleus accumbens. These neurons are normally responsible for inhibiting the reward pathway, but the presence of cannabis stops them from functioning normally. This causes an increase in activity in the reward pathway of the brain, realized as increased dopamine levels (Gardener). However, it must be taken into account that there are CB1 receptors on Glutaminergic interneurons in these regions as well. These neurons normally excite the reward pathway, so when cannabis stops them from functioning properly, there is less activity in these brain areas. Therefore, any dis-inhibition of dopamine neurons caused by cannabinoid action on GABAergic neurons must be balanced with the inhibiting effects caused by their action on Glutaminergic neurons (Gardner). Furthermore Leeca et. al., who measured extracellular levels of dopamine in the nucleus accumbens of rats with the injection CB1 receptor agonists found that dopamine levels only increase to approximately 150% of basal rates (Leeca et. al.). According to the NIDA, this minor increase in dopamine levels is identical to the increases in dopamine seen from eating food (Chiaria et. al.). Even though the mechanisms of food and drug addiction are slightly different, I argue that the majority of humans arecapable of cognitively overriding impulses to over consume fatty or sugary foods. Therefore it should at least be taken into account that previously mentioned studies on cannabis self-administrationrates have been conducted in animal models with underdeveloped and environmentally under stimulated prefrontal cortices who are more likely to impulsively administer the drug than humans might be. So, studies that claim that cannabis self-administration in animals is good evidence that the drug is addicting in humans are making presumptuous and unsupported leaps. Not coincidentally, many of the studies that do report dependence on marijuana in humans only cite children as being prone to addiction, even though it is widely known that underdeveloped areas in the left portion of the frontal cortex leave them more susceptible to addiction (Wieland et. al.). Before it can be concluded that cannabis has threating abuse potential for adult medical patients, more studies need to be done about its rewarding effects in adult humans, who have a greater ability to consciously override minimal physiological withdrawal symptoms than animals or children.
overriding impulses to over consume fatty or sugary foods. Therefore it should at least be taken into account that previously mentioned studies on cannabis self-administrationrates have been conducted in animal models with underdeveloped and environmentally under stimulated prefrontal cortices who are more likely to impulsively administer the drug than humans might be. So, studies that claim that cannabis self-administration in animals is good evidence that the drug is addicting in humans are making presumptuous and unsupported leaps. Not coincidentally, many of the studies that do report dependence on marijuana in humans only cite children as being prone to addiction, even though it is widely known that underdeveloped areas in the left portion of the frontal cortex leave them more susceptible to addiction (Wieland et. al.). Before it can be concluded that cannabis has threating abuse potential for adult medical patients, more studies need to be done about its rewarding effects in adult humans, who have a greater ability to consciously override minimal physiological withdrawal symptoms than animals or children.
Other research that describes the effects of various chemical compounds in cannabis support that particular strains of the plant may reduce the addicting properties of other drugs as well as provide their own specific treatment benefits, making them appropriate for aggregate use with other drugs. Firstly, THC has been suggested as an appropriate aggregate of opioid painkillers because of a synergistic pain relieving effect when the two are combined (Lucas). This is a potentially fruitful use for cannabis because opioid painkillers are among the most abused prescription drugs in the United States. Because both drugs are pain relievers, a smaller dose of each can be used when mixed to achieve the same overall effect. But beyond that, the synergistic effects when combined cause both the THC and the opioid components to become more potent. Therefore the dose of each can be lowered even further than expected. 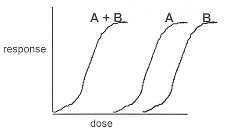 By lowering the required amounts of of each so dramatically to treat patients’ symptoms, the possibility of both addiction and the severity of other negative side effects associated with either drug can be reduced. Furthermore, not only does THC create a synergistic analgesic effect in combination with opioids, but its aforementioned non-psychoactive compound, cannabidiol, has been shown to create a drop in the addictive properties of opiates when the two are used in conjuction with one another (Prud’homme et. al.). Thus, a lowering of the opiate dose and an additional reduction of the effectiveness of the drug’s addictive properly could greatly reduce the potential for addiction. By extension, strains of cannabis with high levels of cannabidiol may serve as an appropriate treatment for patients with narcotic drug addictions formed through recreational abuse through similar mechanisms of action. This is significant because cannabis could help reduce addictions to heroin, which is becoming one of the leading causes of drug related deaths in the United States (Overdose).
By lowering the required amounts of of each so dramatically to treat patients’ symptoms, the possibility of both addiction and the severity of other negative side effects associated with either drug can be reduced. Furthermore, not only does THC create a synergistic analgesic effect in combination with opioids, but its aforementioned non-psychoactive compound, cannabidiol, has been shown to create a drop in the addictive properties of opiates when the two are used in conjuction with one another (Prud’homme et. al.). Thus, a lowering of the opiate dose and an additional reduction of the effectiveness of the drug’s addictive properly could greatly reduce the potential for addiction. By extension, strains of cannabis with high levels of cannabidiol may serve as an appropriate treatment for patients with narcotic drug addictions formed through recreational abuse through similar mechanisms of action. This is significant because cannabis could help reduce addictions to heroin, which is becoming one of the leading causes of drug related deaths in the United States (Overdose).
Another main concern with medical marijuana, that short term cognitive side effects may hinder the patients’ ability to function in daily activities, has more merit than previous dissenting arguments but still does not account for side effects in scope of the treatment benefits. There is some legitimacy to claims made about short-term cognitive impairment in patients. Some studies have shown that the decrease in Glutamine release caused by activation of CB1 receptors by the active ingredients in cannabis can hinder the process of long-term-potentiation that is frequently utilized by the brain in learning. As a result, over active CB1 receptors in the hippocampus and the frontal cortex can cause both memory deficits and some mild decision-making and planning impairments in the short term. Other short-term cognitive side effects of cannabis include dizziness, drowsiness, and anxiety (Lamorine). But in many situations the positive effects associated with cannabis administration could be so great that the side effects are negligible. For instance, recent studies have shown that children who use cannabis as a treatment for early onset seizure disorders actually show improvement in their cognitive functioning after cannabis administration (Hussain et. al.). In his documentary, Weed, CNN’s chief medical correspondent Dr. Sanjay Gupta shows an example of a female child, Charlotte Figi, that goes from having hundreds of seizures per week pre-cannabis treatment to fewer than 10 seizures per week afterwards. For the first time she is able to utilize her own cognitive function without being in a postictal cognitive state from all the seizures (Gupta). What’s more, this lack of side effects may also be because the cannabidiols needed for the treatment of movement disorders do not produce the same psychoactive high and side effects of THC (Porter and Jaobson). By and large, other disorders that would utilize mainly the CBD of cannabis such as schizophrenia and Parkinson’s, could use it as an effective treatment with barely any short term side effects. Plus, concerning long term side effects, evidence about the way in which patients build a tolerance to cannabis has revealed that long-term cannabis users become tolerated to cognitive side effects of the drug that inhibit processes of learning, but not the psychoactive effects that are responsible for treating ailments (Dellate et. al.). This means that marijuana could be an appropriate form of long-term treatment of certain disorders because it won’t cause cognitive inhibition after extended use. Not only that, but it could be more beneficial to the patients because they won’t have to alter dosages much in the long run. They could spend less money and time dealing with their treatment and focus more on their daily activities. So once again, an appropriate combination of cannibidiols and THC could allow for drugs to be created that have life altering effects with minimal short and long-term side effects.
Even though medical cannabis has many potentially beneficial uses that rival and even outweigh those of other prescription medications, the individual studies being performed on cannabis seem to be focusing on the wrong goals. Many studies cast marijuana in a negative light and aim to show its harmful properties as a casual drug of abuse. But, by conducting more research on specific strains of cannabis that have treatment potential for very specific illnesses, it may be found that cannabis has as many or more positive applications and many fewer side effects than anticipated.
For a comprehensive list of Medical Marijuana Laws by state, click here.
References
Chiara, G. D. (1999). Drug addiction as dopamine-dependent associative learning disorder. European Journal of Pharmacology, 375(1-3), 13-30. doi:10.1016/s0014-2999(99)00372-6
Conway, D., & Cohen, J. A. (2010). Combination therapy in multiple sclerosis. The Lancet Neurology, 9(3), 299-308. doi:10.1016/s1474-4422(10)70007-7
DEA / Drug Scheduling. (n.d.). Retrieved August 04, 2015, from http://www.dea.gov/druginfo/ds.shtml
Delatte, M., Winsauer, P., & Moerschbaecher, J. (2002). Tolerance to the disruptive effects of Δ9-THC on learning in rats. Pharmacology Biochemistry and Behavior, 74(1), 129-140. doi:10.1016/s0091-3057(02)00966-8
Gardner, E. (2005). Endocannabinoid signaling system and brain reward: Emphasis on dopamine. Pharmacology Biochemistry and Behavior, 81(2), 263-284. doi:10.1016/j.pbb.2005.01.032
Goodman, J., & Packard, M. G. (2015). The influence of cannabinoids on learning and memory processes of the dorsal striatum. Neurobiology of Learning and Memory, 125, 1-14. doi:10.1016/j.nlm.2015.06.008
Gupta, S. (n.d.). Sanjay Gupta: Time for a medical marijuana revolution – CNN.com. Retrieved August 05, 2015, fromhttp://www.cnn.com/2015/04/16/opinions/medical-marijuana-revolution-sanjay-gupta/
Hussain, S. A., Zhou, R., Jacobson, C., Weng, J., Cheng, E., Lay, J., . . . Sankar, R. (2015). Perceived efficacy of cannabidiol-enriched cannabis extracts for treatment of pediatric epilepsy: A potential role for infantile spasms and Lennox–Gastaut syndrome. Epilepsy & Behavior, 47, 138-141. doi:10.1016/j.yebeh.2015.04.009
Iseger, T. A., & Bossong, M. G. (2015). A systematic review of the antipsychotic properties of cannabidiol in humans. Schizophrenia Research, 162(1-3), 153-161. doi:10.1016/j.schres.2015.01.033
Justinova, Z., Tanda, G., Redhi, G. H., & Goldberg, S. R. (2003). Self-administration of ?9-tetrahydrocannabinol (THC) by drug naive squirrel monkeys.Psychopharmacology, 169(2), 135-140. doi:10.1007/s00213-003-1484-0
Jutras-Aswad, D., Prud’Homme, M., & Cata, R. (2015). Cannabidiol as an Intervention for Addictive Behaviors: A Systematic Review of the Evidence. SART Substance Abuse: Research and Treatment, 33. doi:10.4137/sart.s25081
Lamarine, R. J. (2012). Marijuana: Modern Medical Chimaera. Journal of Drug Education, 42(1), 1-11. doi:10.2190/de.42.1.a
Lecca, D., Cacciapaglia, F., Valentini, V., & Chiara, G. D. (2006). Monitoring extracellular dopamine in the rat nucleus accumbens shell and core during acquisition and maintenance of intravenous WIN 55,212-2 self-administration. Psychopharmacology, 188(1), 63-74. doi:10.1007/s00213-006-0475-3
Lucas, P. (2012). Cannabis as an Adjunct to or Substitute for Opiates in the Treatment of Chronic Pain. Journal of Psychoactive Drugs, 44(2), 125-133. doi:10.1080/02791072.2012.684624
Mcallister, S. D., Soroceanu, L., & Desprez, P. (2015). The Antitumor Activity of Plant-Derived Non-Psychoactive Cannabinoids. Journal of Neuroimmune Pharmacology J Neuroimmune Pharmacol, 10(2), 255-267. doi:10.1007/s11481-015-9608-y
Naef, M., Curatolo, M., Petersen-Felix, S., Arendt-Nielsen, L., Zbinden, A., & Brenneisen, R. (2003). The analgesic effect of oral delta-9-tetrahydrocannabinol (THC), morphine, and a THC-morphine combination in healthy subjects under experimental pain conditions. Pain, 105(1), 79-88. doi:10.1016/s0304-3959(03)00163-5
Overdose Death Rates. (n.d.). Retrieved from http://www.drugabuse.gov/related-topics/trends-statistics/overdose-death-rates
Porter, B. E., & Jacobson, C. (2013). Report of a parent survey of cannabidiol-enriched cannabis use in pediatric treatment-resistant epilepsy. Epilepsy & Behavior, 29(3), 574-577. doi:10.1016/j.yebeh.2013.08.037
Rog, D. J., Nurmikko, T. J., Friede, T., & Young, C. A. (2005). Randomized, controlled trial of cannabis-based medicine in central pain in multiple sclerosis.Neurology, 65(6), 812-819. doi:10.1212/01.wnl.0000176753.45410.8b
Weiland, B. J., Korycinski, S. T., Soules, M., Zubieta, J., Zucker, R. A., & Heitzeg, M. M. (2014). Substance abuse risk in emerging adults associated with smaller frontal gray matter volumes and higher externalizing behaviors. Drug and Alcohol Dependence, 137, 68-75. doi:10.1016/j.drugalcdep.2014.01.005

Leave a Reply