In recent weeks, numerous reports and presentations have been published by NIH and the Director of the Office of Science and Technology Policy on principled international collaborations, protecting research against foreign influence, and ensuring the security and objectivity of research. NIH has a website specifically addressing these issues entitled Protecting U.S. Biomedical Intellectual Innovation. NSF has also updated the Proposal & Award Policies & Procedures Guide (PAPPG) and clarified which relationships, affiliations, and resources must be disclosed under biographical sketch and the current and pending support through a Frequently Asked Questions guide. These reports and resources may be found at the Emory Research webpage on International Collaborations.
Emory continues to be unwavering is in its support of international collaboration with our research partners around the world. Researchers must be fully transparent about their financial support and resources from and affiliations with international institutions and ensure that this information is updated throughout the life of a federal award.
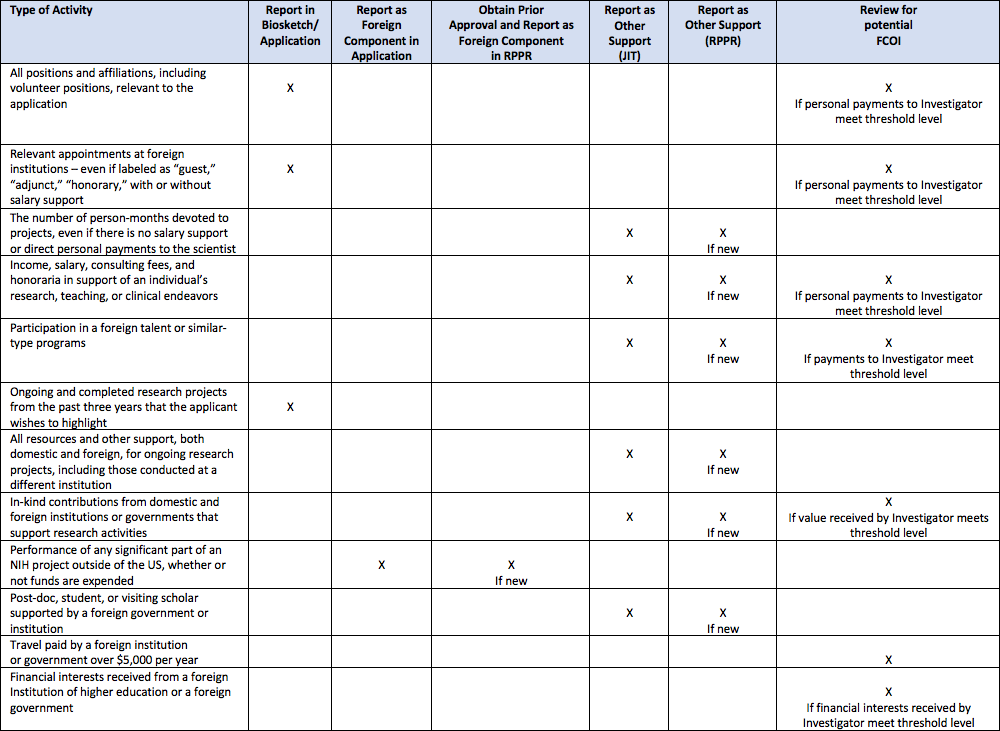
For NIH, the disclosure of all sources of research support, prior approval of all foreign components, and appropriate reporting and management of financial conflicts of interest is required. The following chart is helpful in identifying the different types of disclosures and under which form disclosure is required:
*Note: Please click on the image to expand.
Under the Emory policy, the threshold levels for FCOI reporting are:
- $5,000 received annually, in aggregate, from for profit or non-profit (US or foreign) entities for services fees, consulting, honoraria, travel and accommodation
- Exclusion from this requirement are:
- Payments from the US federal, state, or local government agencies
- Payments from US Institutions of higher education
- Payments from US teaching hospitals
- Payments from research institutes affiliated with US Institutions of higher education
- Exclusion from this requirement are:
- Holding equity interests in a publicly traded company that exceeds $5,000 in value
- Holding any ownership/equity interest in a privately held company
- Holding a fiduciary role in a for profit entity
- Licensing fees or royalties that exceed $5,000 annually
Under the Emory Faculty Handbook, all faculty are required to disclose all external activities such as consulting and advisory roles prior to engagement and obtain prior approval from your Department Chair and Dean’s Offices. Additionally, teaching, research, or service at other institutions must also be disclosed, reviewed and approved by the Department Chair and Dean. These disclosures may be made through eCOI system, which automatically forwards the information to the Department Chair and Dean for approval.