Danial Arslan
Social interactions are often thought to be one of the most basic human needs that exists, alongside the need to find food to eat and water to drink. Some even postulate that our brains evolved, over the millennia, due to selection pressures placed because of the social environments our ancestors lived in. This came to be known as the social brain hypothesis.
As it turns out this instinctive need to form social bonds transcends the human species.
Studies have found genes which regulate social behaviours in distant organisms like the Drosophila melanogaster, Caenorhabditis elegans and Microtus ochrogaster. Thus, it can be inferred that even these species have their own systems of social hierarchies, societies and social bonds.
A variety of neuropsychiatric disorders such as schizophrenia and autism spectrum disorders are characterized by disruptions in these social behaviours and bonds. Hence, there has been a recent surge in the study of the neural circuitry involved in forming social bonds in order to better understand these diseases. Since social bonds aren’t only restricted to humans, we can study these disorders by studying neural pathways involved in forming bonds in other species first.
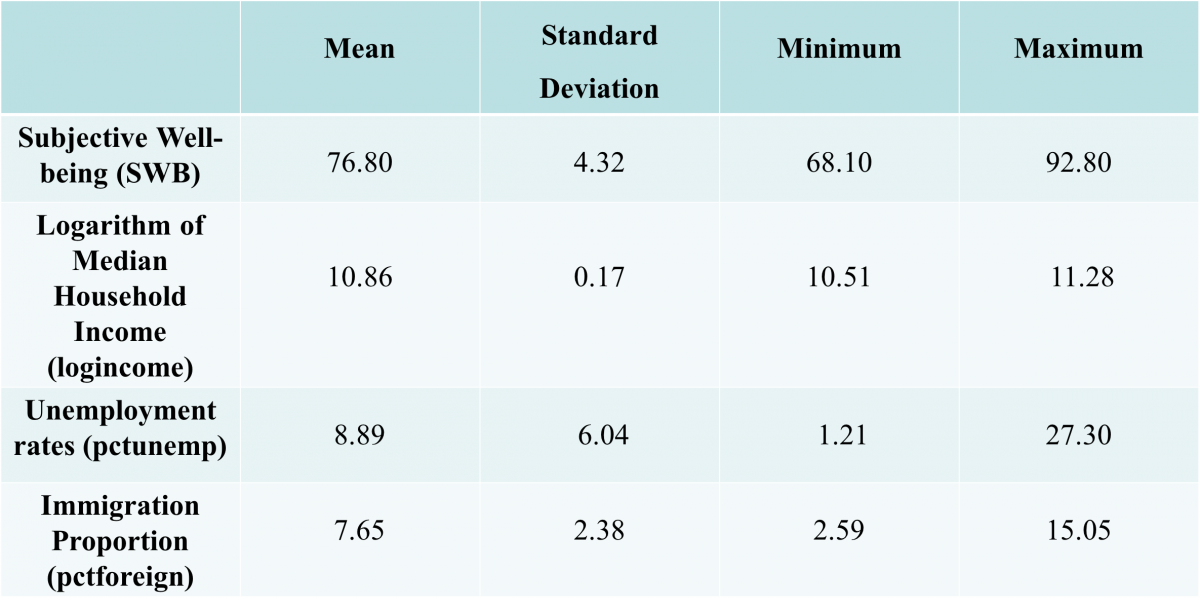
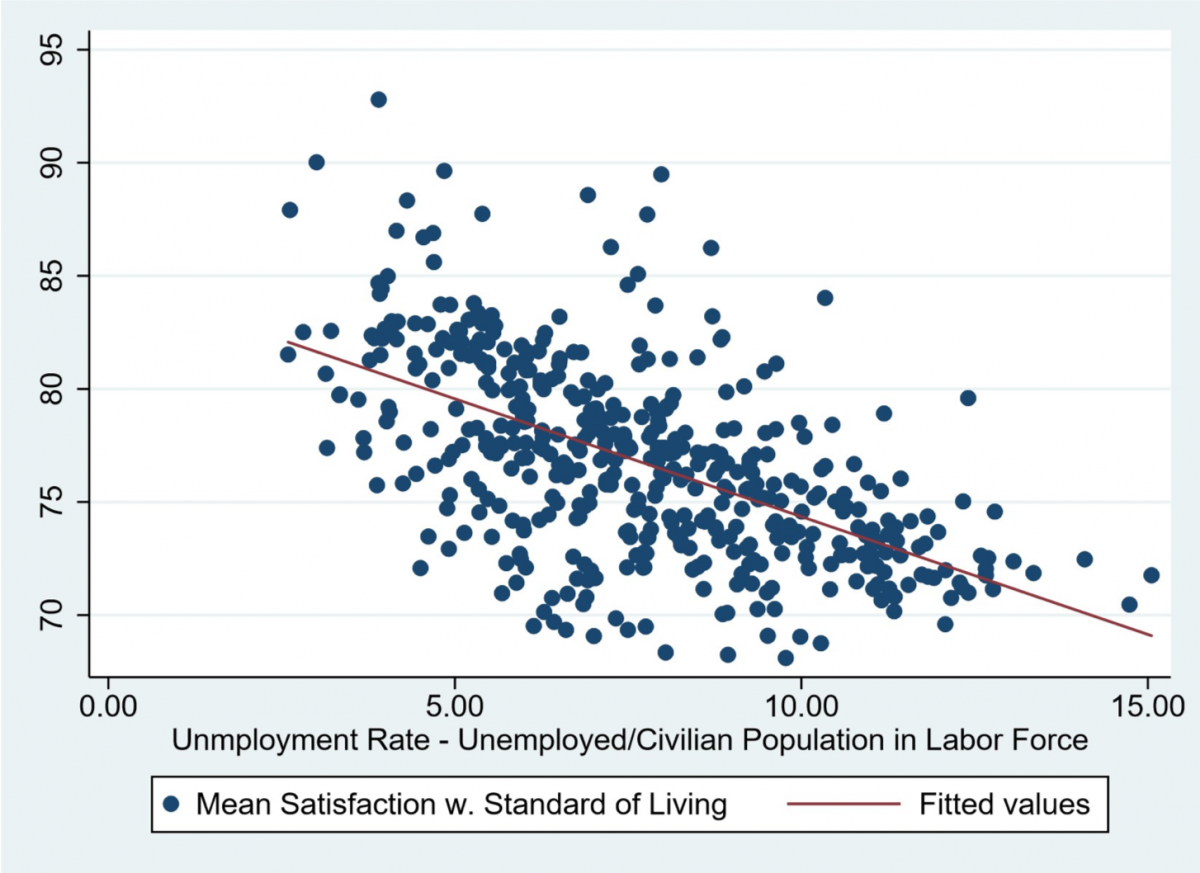
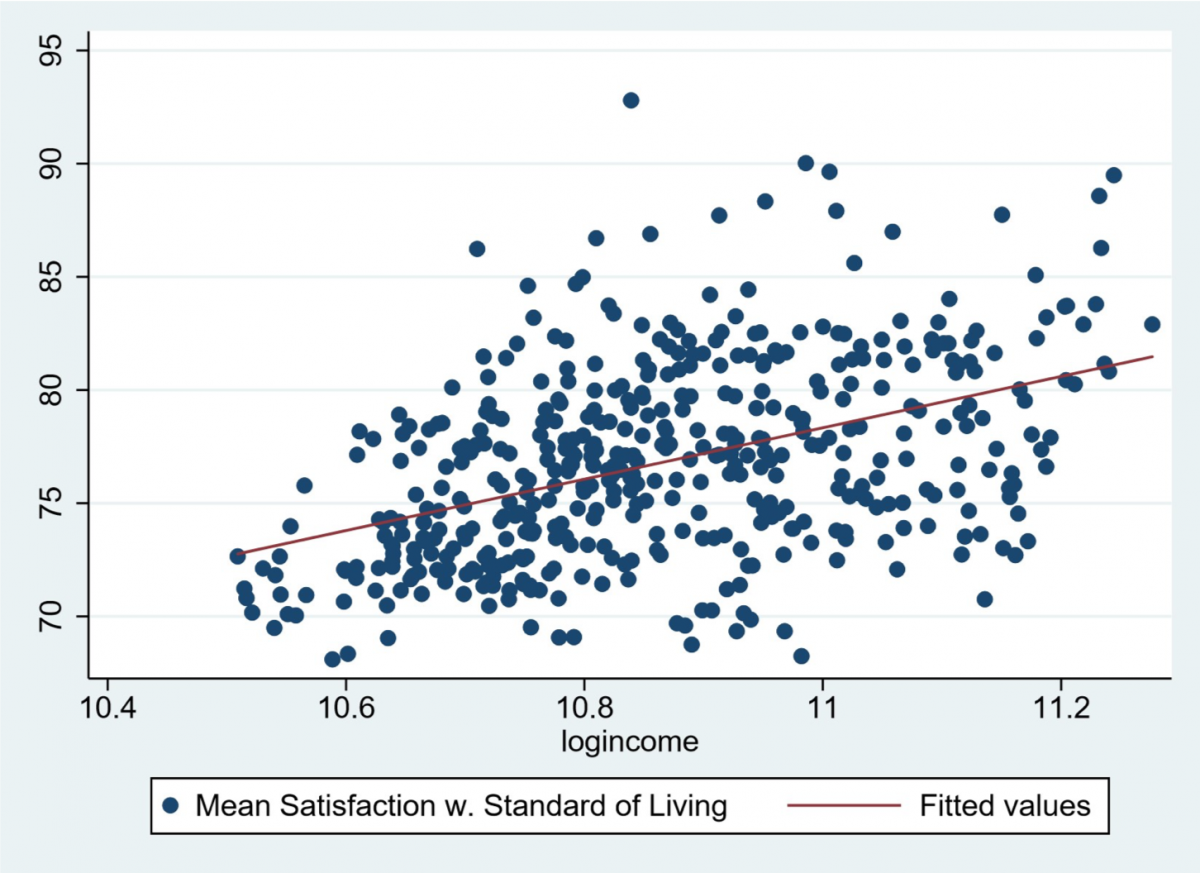
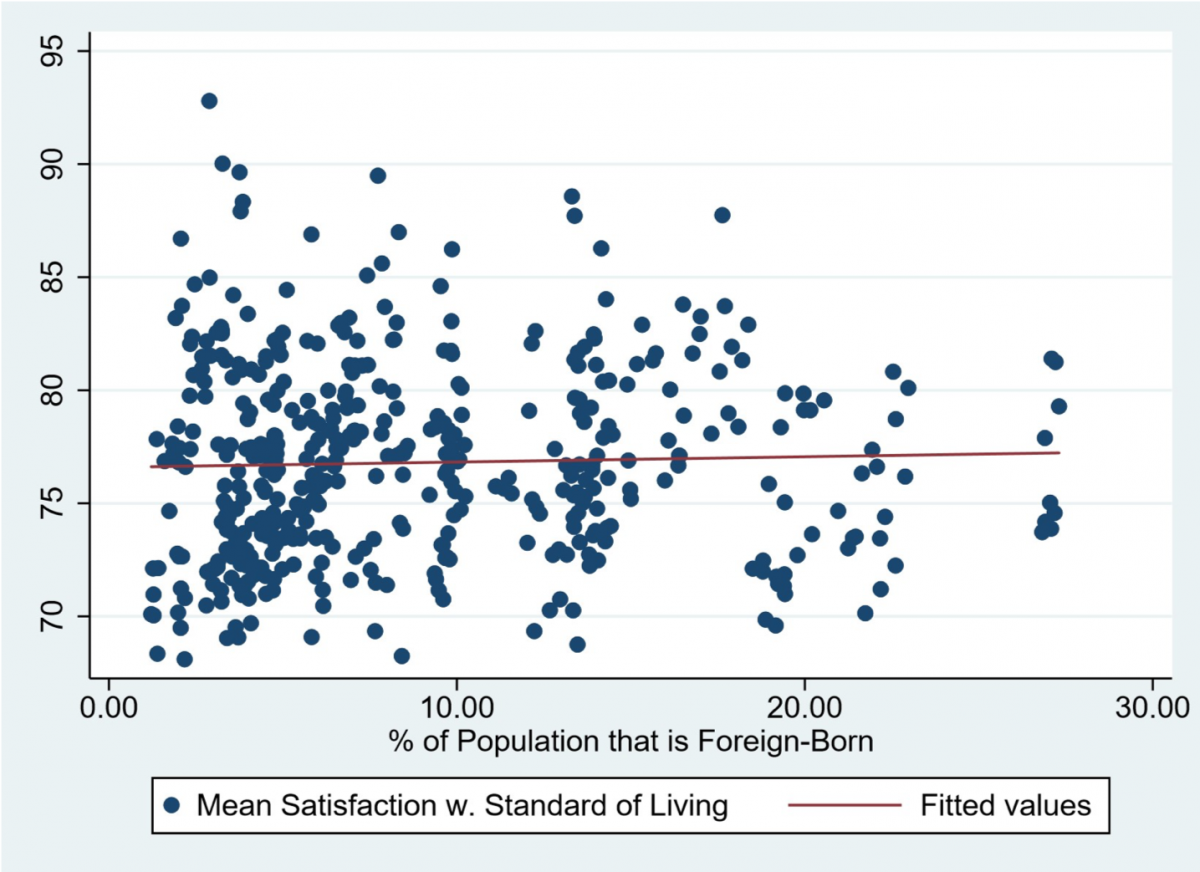
My lab has been studying prairie voles (Microtus ochrogaster) due to their surprisingly monogamous nature (as described in my previous blog post here). Based on previous work done, the lab is trying to ascertain whether oxytocin or specific brain waves, between the nucleus accumbens and the medial prefrontal cortex regions of the brain, might be responsible for the formation of these monogamous relationships.
The current data which led to this finding has been based on analysing brain waves, alongside affiliative behaviours seen, while voles were cohabitating. However, in order to find a stronger correlation between our independent variables (oxytocin concentration or the frequency of brain waves between the two brain regions) and what we wanted to measure experimentally (the strength of the partner bond formed) we needed to devise a more straightforward test and this is what I and one of the graduate students in my lab, sought out to do.
And so after weeks of testing different theories, we came up with a 2 way odour expoure test that we will now use moving forward. The basic principle would be to ‘entrap’ the scent of two female voles. We did this by collecting the bedding used by the voles while singly caged. (Side note: Since vole’s love making burrows paper bedding is placed in their cages to keep them busy). This paper bedding successfully captured their smells and was added to a petri dish with an exact number of perforations on the top and bottom of the dish’s cover. This would allow the scent to be carried as air passed in through one set of perforations and moved out of the other.
After entrapping the female scent, one female would be cohabitated with the male vole to establish the pair bond and then the male vole would be placed in a cage with 3 compartments as shown in the diagram below:
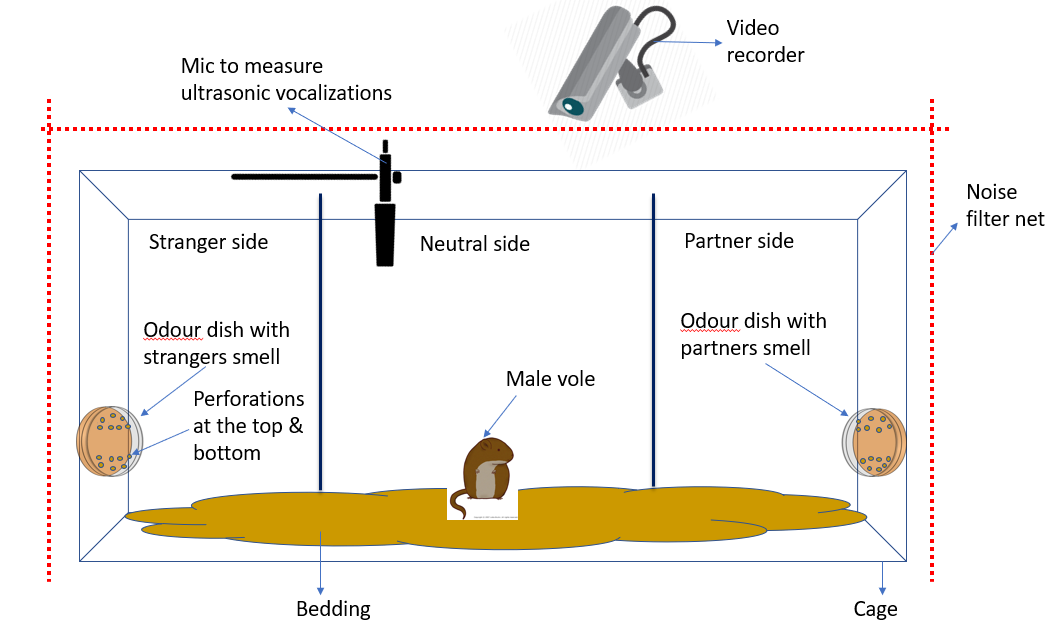
The ‘partner’ would be the female the male was housed with and the ‘stranger’ would be the female who was never housed with the male. The male would be placed in the center compartment and his movement to different sides would be tracked.
Then after the assay, the time spent in each compartment would be analysed, as well as the time spent by the vole sniffing and investigating each of the petri dishes, to ascertain whether the vole preferred the partner compartment and petri dish over the other one.
Meanwhile, any vocalizations made by the vole in these different sides would also be recorded to see if the vole’s vocalizations differed when it was in a side of the cage where it could smell its partner to when it was in the stranger side.
A general outline of the entire procedure can be seen with this schematic:
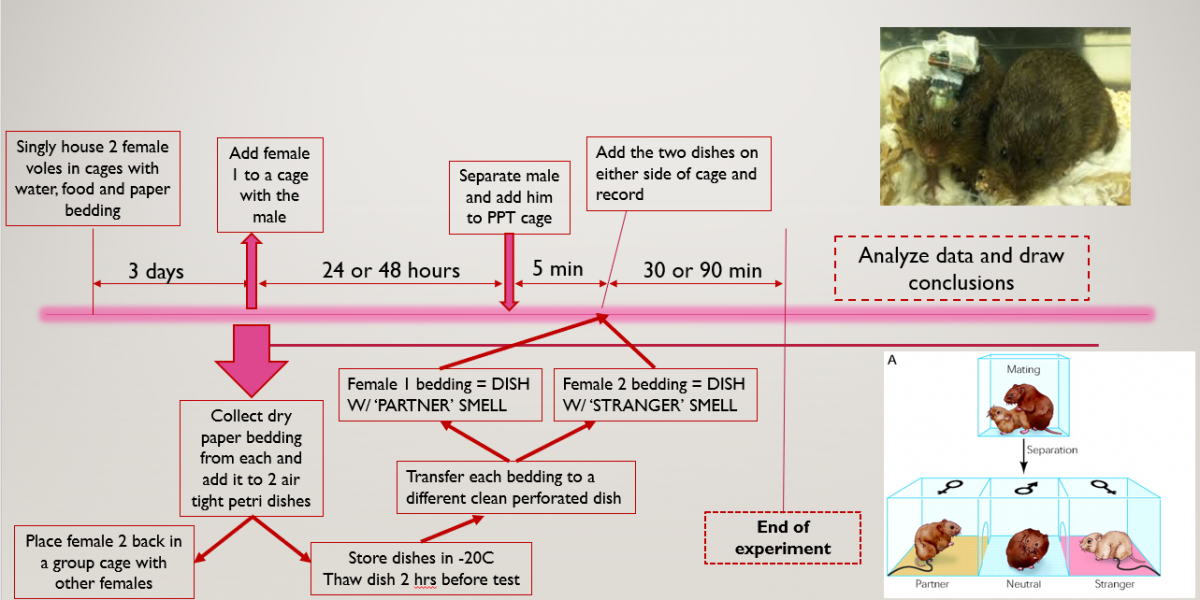
And so, we came up with an odour exposure test which would be done by seeing which odour the vole would investigate more. The basic assumption was that the vole would deduce that the smell of the female vole would indicate her presence and so would call out to her. We tweaked around the time we cohabitated the voles for as well as the time of the partner preference test itself to see under what conditions we could obtain the best data and that would be used moving onwards.
So, in conclusion, our odour exposure test would look at how the male vole reacts and subsequently behaves to the presence of olfactory cues which give it the impression that it is close to its partner. Preference would be seen by looking at time spent in a particular side of the cage, time spent sniffing the petri dish and auditory calls made out when in a particular side. This data could then be used to see if the behaviours do in fact differ when the vole detects the partner scent or the stranger scent. If a significant difference is seen, then in the future a neurologger device could be inserted into the brain regions of interest to see the exact brain waves and levels of oxytocin present during these behaviours and hence this devised test could act as a standardized assay for future experiments in the field.