The following post will describe an update on my project to someone outside of academia. I am currently analyzing a channel located within the mitochondria. The channel is called the Mitochondrial Calcium Uniporter (MCU). MCU is highly selective to Calcium and only allows passage of calcium into the mitochondria.
My lab is studying this specific selectivity for calcium in hopes to better understand the MCU functionality and find better ways to regulate such a channel. Currently, we have identified a subunit located on the MCU called MCUb. It has been proven that over expression of this subunit prohibits the flow of calcium through the channel. However, upon further examination, we discovered that there are few regions between the two that are different. The regions in which we are analyzing is called the motif regions. These regions are the ones lining the inside of the channel and the ones responsible for the high calcium selectivity.
Currently, we are creating mutations of the MCU based upon the differences of the motif regions. These mutations are specifically designed to aid us in narrowing down the specific changes and modifications that result in the changes that we see between MCU and MCUb. To analyze these changes we manually add calcium sensitive fluorescence into the mitochondria. With this fluorescence we are able to track the intake of calcium into the mitochondria through the MCU by measuring the increase of intensity of fluorescence over time. Through calculations and analysis, we can use our data to directly calculate intake of calcium for different mutations our lab creates.
I have currently examined 4 cell lines each with a different mutation. We are still analyzing the results, but we have seen some positive data indicating that these mutations are causing alterations to how calcium is entering the mitochondria.
With time, I hope to finish up the data analysis and continue to image more cell lines with mutations. One major bottleneck that my project is experiencing is the creation of the mentioned mutated cell lines. Creating stable cell lines with the specific mutations that we desire takes a considerable amount of time and we are currently working on creating as many cell lines as possible so that we can get a wide range of mutations to test. However, we hope to have enough mutations that we can narrow down the specifics of how we can better regulate the Mitochondrial Calcium Uniporter.

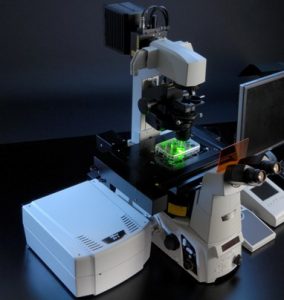 Over the past couple of weeks, I have been facing my ultimate nemesis in my project, confocal microscopy. Confocal microscopy is a microscope that is able to measure fluorescence (light) from a fluorescent dye (light inducing). In the beginning, I thought it was going to be an easy task, but I was deeply mistaken. It has taken my a total of 5 weeks to master the microscope and to produce usable data for my project. The main hurdle that I encountered was learning to permeabilized my cells. This was a crucial step because it was going to yield accurate and useful data. If I didn’t master this, my whole project would come falling down.
Over the past couple of weeks, I have been facing my ultimate nemesis in my project, confocal microscopy. Confocal microscopy is a microscope that is able to measure fluorescence (light) from a fluorescent dye (light inducing). In the beginning, I thought it was going to be an easy task, but I was deeply mistaken. It has taken my a total of 5 weeks to master the microscope and to produce usable data for my project. The main hurdle that I encountered was learning to permeabilized my cells. This was a crucial step because it was going to yield accurate and useful data. If I didn’t master this, my whole project would come falling down.
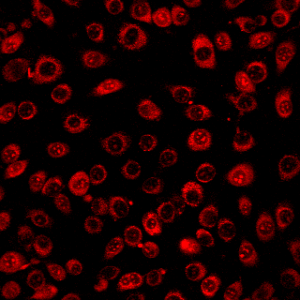 From what i achieved, a successful permeabilization will result in a grainy texture of the cell fluid that surrounds the nucleus. The grainy texture is in fact small bubbles that are a result of the break down of the cell membrane. Once we reach this stage, the permeabilization has just begun. To make sure that it is the correct amount permeabilization, the bubbles will increase in size and become even more grainy. Eventually, there comes a point where the bubbles are just the right size so that it will not destroy the membrane and it’s big enough to allow dye to flow out and calcium to flow in. This point is up to the discretion of the researcher. There is not a justified size to the bubbles, but after many practice rounds, there is a preferred size for each researcher every time.
From what i achieved, a successful permeabilization will result in a grainy texture of the cell fluid that surrounds the nucleus. The grainy texture is in fact small bubbles that are a result of the break down of the cell membrane. Once we reach this stage, the permeabilization has just begun. To make sure that it is the correct amount permeabilization, the bubbles will increase in size and become even more grainy. Eventually, there comes a point where the bubbles are just the right size so that it will not destroy the membrane and it’s big enough to allow dye to flow out and calcium to flow in. This point is up to the discretion of the researcher. There is not a justified size to the bubbles, but after many practice rounds, there is a preferred size for each researcher every time.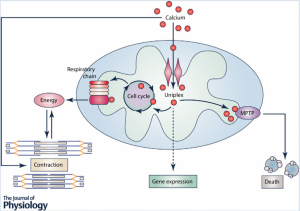 Image from Kwong (2015)
Image from Kwong (2015)