click the box below to complete the ARISE interest survey!
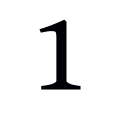
Are there any risks associated with participating in this study?
Yes, some risks in blood collection include fainting, bruising, soreness, tenderness at the needle site, infection, blood clot, bleeding from the puncture site, and swelling of the vein and surrounding tissue.
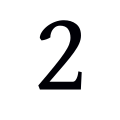
How long will this study last?
The study will last a total of 14 days for participants.
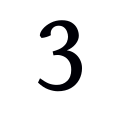
Do I have to live in the metro Atlanta area to participate?
Yes, all participants must live in the metro Atlanta area.
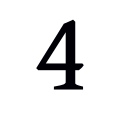
Will the devices track my location even if I’m not asleep?
Yes, the study employs one app that tracks your location throughout the day. Within the app and our records, your location will not be traced back to you.
Will my information ever be sent out to other people?
Information is kept confidential and only seen within the research team.
Will I be compensated for my participation?
Yes, each participant is eligible to receive up to $500 for completing this study.
Do I have to pay anything to be a part of this study?
No, participants do not have to pay to be part of this study. All tests, devices, and transportation costs will be covered or compensated.
Will I need to change my routines if I participate in this study?
Yes, participants will attend 1 clinic visit at Emory University involving blood pressure measurement and blood collection, and 5 to 6 home visits involving saliva collection and a questionnaire. Participants will wear devices to measure sleep and sleep apnea. Participants will also place devices around their home for 14 days to measure air quality, light exposure, noise level, and temperature. Participants should avoid travel plans outside of the metro Atlanta area during the study.

