Authors: Kathryn M. Chepiga †, Yan Feng ‡, Nicholas A. Brunelli ‡, Christopher W. Jones *‡, and Huw M. L. Davies *†
Organic Letters
2013, 15 (24), pp 6136–6139.
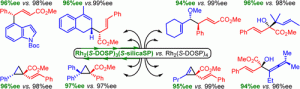
A silica-supported dirhodium(II) tetraprolinate catalyst was synthesized in four steps from l-proline and used in a range of enantioselective transformations of donor/acceptor carbenoids. These include cyclopropenation, cyclopropanation, tandem ylide formation/[2,3] sigmatropic rearrangement, and a variety of combined C–H functionalization/Cope rearrangement reactions. The products of these transformations were obtained in yields and levels of enantioselectivity comparable to those obtained with its homogeneous counterpart, Rh2(S-DOSP)4. The silica-supported Rh2(S-DOSP)4 derivative was successfully recycled over five reactions.