Projects
Cu-catalyzed C-O cross-coupling reaction for the synthesis of structurally complex vinyl ethers
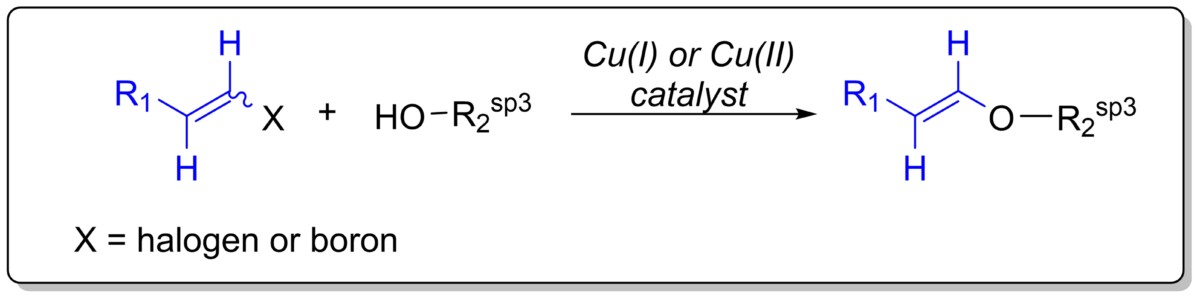
Vinylic ethers are versatile compounds, utilized as reactive intermediates or precursors in total syntheses and synthetic methods including bio-orthogonal reactions. Current technologies are limited in scope and unreliable, therefore, we aim to develop a general approach to produce structurally complex vinyl ethers.
We are currently working on two approaches to synthesize these compounds: 1) Cu(I)-catalyzed Ullmann-type cross-coupling reaction with (E) or (Z)-vinyl iodide and complex aliphatic alcohols; 2) Cu(II)-catalyzed Chan-Evans-Lam-type cross-coupling reaction with (E) or (Z)-vinyl organoboron and complex aliphatic alcohols.
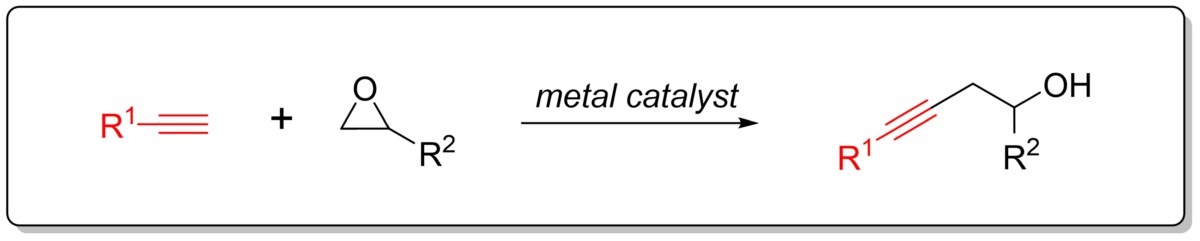
Epoxide ring opening with terminal alkyne for the synthesis of homopropargylic alcohol:
We aim to develop a mild and efficient method of forming homopropargylic alcohol through a transition metal-catalyzed epoxide ring opening reaction with alkyne via formation of a metal acetylide.
Medicinal chemistry:
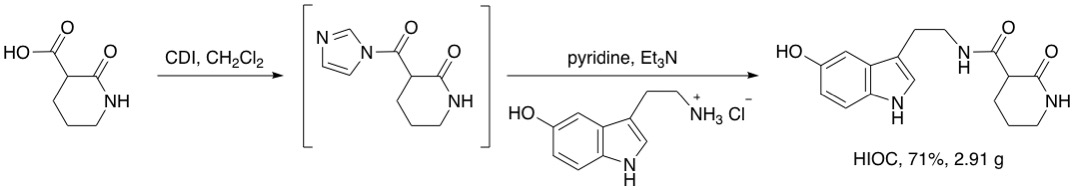
In collaboration with laboratories from Emory School of Medicine and Clark Atlanta University respectively, we are currently developing novel drug candidates for treatments of ocular blast-induced vision loss and prostate cancer.
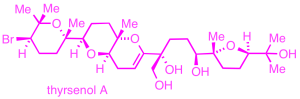
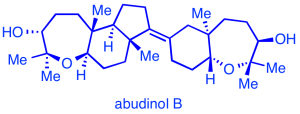
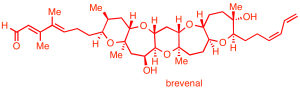
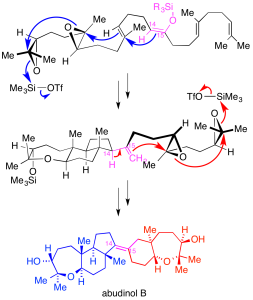
Polycyclic ether natural products:
We aim to establish efficient synthetic routes to a variety of cyclic and polycyclic ether marine natural products, represented by the triterpenes thyrsenol and abudinol (anticancer activity), as well as brevenal (blocks neurotoxic activity of red tide toxins).
Our synthetic approaches feature regio- and stereoselective oxacyclization transformations, including a preparation of abudinol B that is inspired by likely biosynthetic pathways.
Acyclic and macrocyclic natural products:
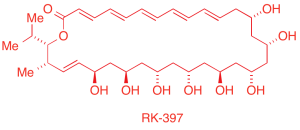
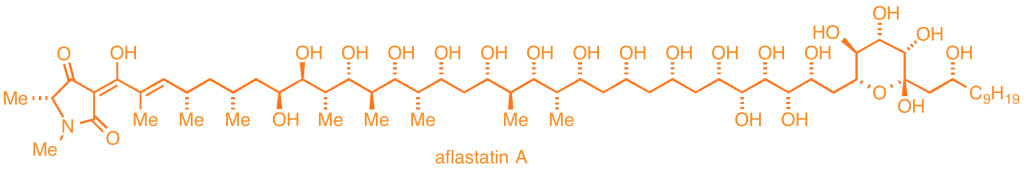
We are developing two mechanistically unique strategies for the preparation of acyclic and macrocyclic natural products. The first program is based on iterative couplings of epoxyalkyne modules, applied to the total synthesis of the anticancer natural product RK-397, as well as a substantial substructure of aflastatin.
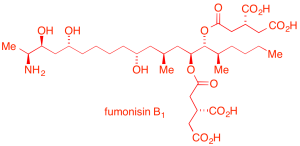
The second program is based on stereospecific pericyclic rearrangements for the simultaneous construction of up to three elements of stereochemistry (one or two chiral centers combined with alkene stereochemistry). This enabling methodology has been applied in preparing two substructures of the sphingolipid biosynthesis inhibitor, fumonisin B1.
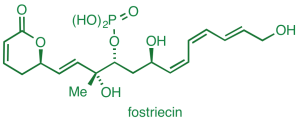
We have also completed a unique synthesis of the anticancer natural product fostriecin.
Oligosaccharides:
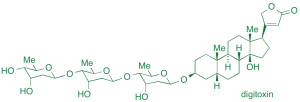
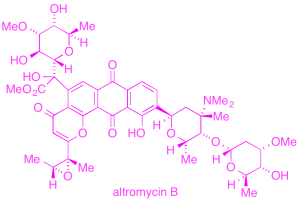
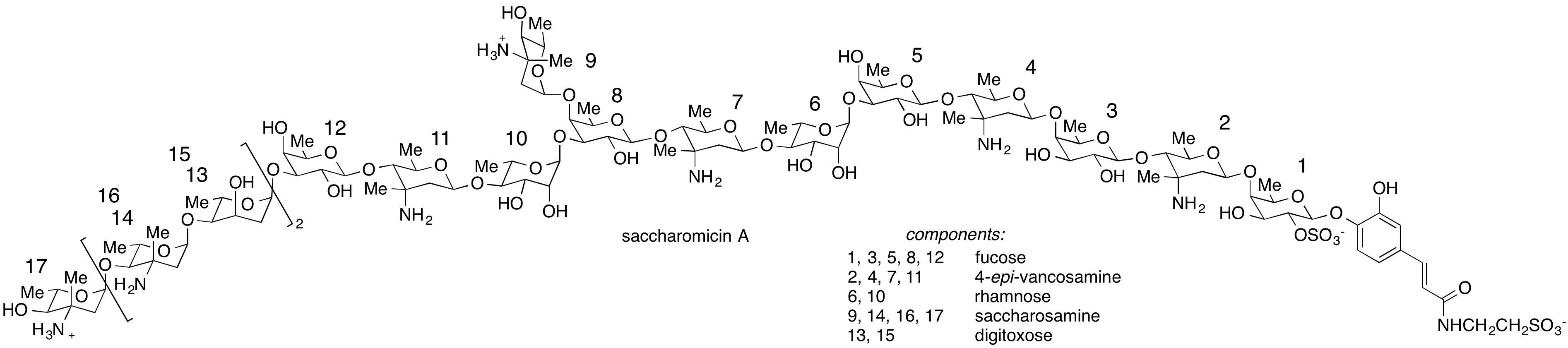
We have invented a mechanistically novel cycloisomerization method for the preparation of cyclic enol ethers, with applications to several oligosaccharide and C-arylglycoside natural products, including the cardiotonic drug digitoxin, an anticancer natural product altromycin, and the antibacterial natural product saccharomicin.
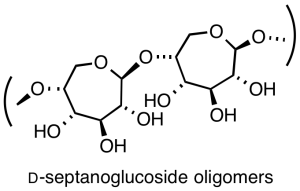
Following up on a serendipitous discovery, we have initiated a program directed toward the preparation of the non-natural oligoseptanoside isomers of cellulose and starch, with potential application as biomaterials.