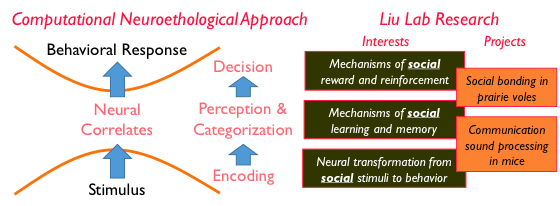
Computational neuroethological framework for studying the neurobiology of social behavior
Computational neuroethology is an interdisciplinary approach to studying the brain. Ethology provides a framework for investigating the functional, mechanistic, developmental, and evolutionary origins of stimulus-elicited natural behaviors. Neuroethology searches for the behaviors’ neural basis. Computational neuroscience enhances this search with quantitative methods for analyzing and modeling complex data sets. Together, computational neuroethology explores neural circuitry and coding by using biologically motivated experiments to reveal correspondences between natural stimulus features, neural activity, and behavior. The approach exploits variation and plasticity in behavior to constrain the neural correlates mediating different stages of processing from encoding through perception and decision. Such correlates reflect putative features of the brain’s activity that could causally drive behavioral responses. Pursuing this strategy in rodents – where modern neuroscience tools can be used to test a mechanism’s necessity or sufficiency – gives us the potential to verify the causal roles of these endogenous correlates.
Neural Coding for Communication in Complex Listening Environments
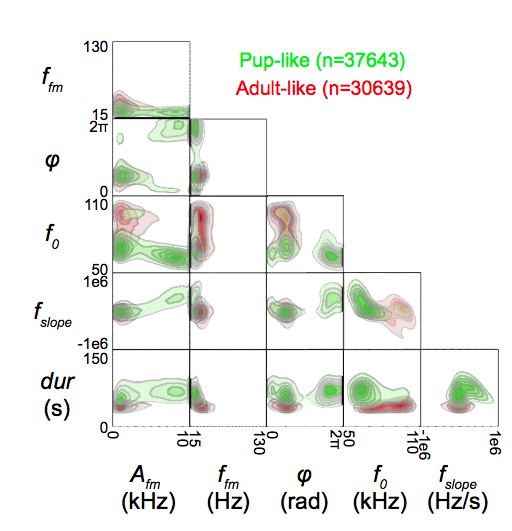
A fundamental task for virtually all auditory systems is to respond to species-specific communication sounds to guide social behavior. What complicates that ability is the fact that communication sounds are naturally variable across multiple acoustic features. For example, mouse ultrasonic vocalizations (USV) are mostly single-frequency whistles that change dynamically over time in both frequency and amplitude. However, just as you would expect for human speech phonemes, no two acoustic waveforms are acoustically identical, with variability from call-to-call, individual-to-individual and context-to-context. Nevertheless, animals can discriminate distinct vocal categories that drive different responses, even when those categories overlap in their acoustic features. How are acoustic waveforms encoded in the auditory system to generate separable category percepts? We use electrophysiological, computational, opto/chemo-genetic and behavioral methods to investigate the coding of USVs within the mouse auditory cortex. Our neural coding studies uncovered a lateral band inhibitory mechanism that can enhance the signal-to-noise in the cortical coding of behaviorally meaningful call categories. We also identified a subset of late-firing neurons in core auditory cortex that spike more for the frequency trajectories of meaningful sound categories compared to those of less meaningful ones. This phenomenon becomes more prominent in the “Off” responses of neurons throughout the secondary auditory cortex. We are now interested in how auditory neural circuits give rise to this sensitivity, and how sensitivity to multiple features can be used to build a neural representation of vocal categories that are tolerant to variability and noise. This capability can then be used to navigate complex listening environments, such as the proverbial cocktail party.
Mechanisms of Social Sound Learning
Acoustic communication disorders impair social interactions, yet how social context interacts with communication sound processing is poorly understood. Like a student in a foreign country immersed in an unfamiliar language or a young mother trying to decipher her baby’s cries, we all encounter initially meaningless vocalizations that in fact convey meaning. As these sounds gain significance through our social interactions, we become better at detecting and discriminating between them. How does this happen? Do our brains learn to associate the sounds with social sources and rewards? Are natural, species-specific vocalizations privileged in how they can drive our social responses? We address these questions using the mouse maternal model of social communication wherein pups emit ultrasonic calls that elicit a mother’s search and retrieval for pups to return them to the nest. We discovered the first electrophysiological signs of sensory plasticity within the auditory system for these calls, encouraging the adoption of this model system to study mechanisms of sensory plasticity in more natural communication contexts. A key question we are now interested in concerns whether this plasticity reflects general associative learning as a cognitive component of motherhood, or may instead be a reflection of an inherent, species-specific trajectory of physiological changes unfolding over the course of motherhood. To address this, we use a T-maze task to pair novel sounds with pup “rewards” to investigate how sensory learning and decision making improves maternal behavior.

Neural Circuitry of Social Bonding
A fundamental question in social neuroscience concerns how social contexts dynamically engage brain areas involved in sensory and reward processing to promote social motivation, learning and attachment. The prairie vole is an excellent model system for studying the neurochemical and neural circuit mechanisms underlying these questions. Prairie voles form socially monogamous pair bonds characterized by both a strong preference to huddle with their partner and a robust defense against strangers. Pair bonding is a complex cognitive process that involves social information processing and learning about the rewarding nature of another individual. Prairie vole research from the lab of our late collaborator, Dr. Larry Young, and others have uncovered key sites of action for neuropeptides like oxytocin or neuromodulators like dopamine to mediate the formation of a pair bond. However, we still do not know the modes of action of these neurochemicals in dynamically modulating neural activity to mediate affiliative behaviors. We demonstrated that a medial prefrontal cortex-to-nucleus accumbens circuit is modulated during affiliative behaviors in a way that is predictive of how quickly voles begin to express their pair bond, and that stimulating this circuit can causally bias the emergence of the bond. We are now investigating how oxytocin mediates signaling in the accumbens and other limbic regions in ways that are critical to bond formation and maintenance.



Quantifying Social Communication in Rodents
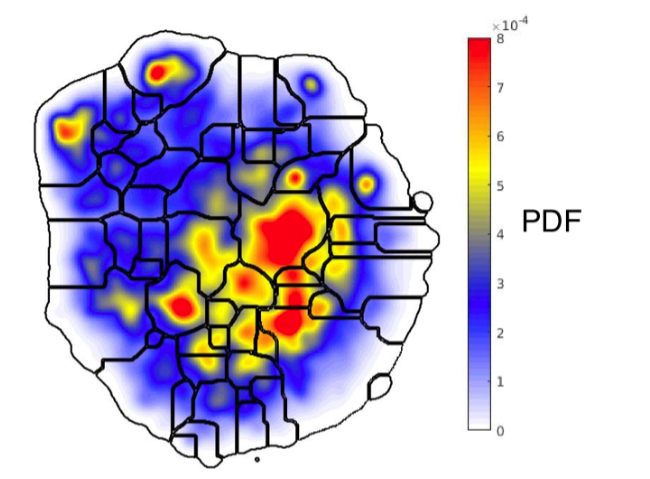
While understanding the neural mechanisms underlying natural behaviors is the main goal of neuroethology, we usually resort to more constrained laboratory paradigms in order to make progress. Even when natural behaviors are used though, we are still often limited to using manual, artificial constructs to describe the behavior, which can lead to interpretations that are biased in ways that are often hidden. To expand our ability to quantify rodent social behavior more automatically in a less biased fashion, we are collaborating with Dr. Gordon Berman to apply machine learning algorithms and quantitative behavioral mapping techniques.
Techniques
Our lab utilizes a variety of techniques, including single-unit and local field potential electrophysiology in awake rodents (mice and prairie voles), multielectrode array electrophysiology, immunohistochemistry, and behavioral analysis. We also make extensive use of computational methods to analyze and model neural data. Our work walks a fine line between developing quantitative engineering approaches and asking novel biological questions.