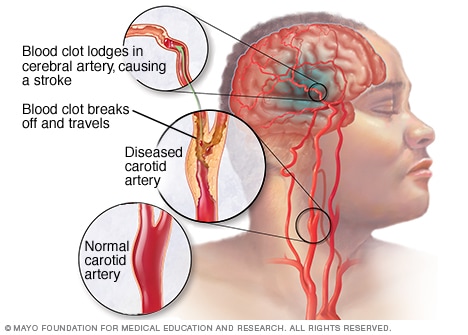
What is Stroke?
Stroke is the leading cause of disability in the United States. The condition causes rapid plasticity within various cortical connections in the brain, essentially causing a remap of neural circuiting in affected areas. When remapping occurs, it forces area of the cortex to form new connections, also known as long-term potentiation. This diminishes responsiveness in certain areas of the brain. In human stroke patients, the damage manifests itself in decreased language, motor, and sensory transfers of information caused by changes in neuronal excitation, which are primarily localized in the somatosensory and primary motor cortices of the brain. 1,2
The heterogeneity of stroke can make it difficult to diagnose, determine prognosis trajectories, and determine an appropriate treatment plan, making the identification of biomarkers8 an imperative tool to determine the level of changes in neural circuitry. However, as far as current research goes, there are no established biomarkers for stroke patients, especially within the context of hemodynamic and neurochemical activity. Whole brain analysis provides significant evidence to suggest a negatively correlative relationship of GABA localized within the primary motor cortex9, indicating that GABA may be a viable biomarker for stroke.1 Additionally, multiple studies have linked decreased levels of GABA in the primary cortex after various non-invasive brain stimulation techniques (NIBS).1,5,8,10
What has previous research shown?
In previous studies, NIBS and PETs techniques were used in order to monitor and record GABAergic activity, however, these do not measure GABA levels directly.1 Magnetic resonance spectroscopy (MRS) has been useful non-invasive techniques have been useful in measuring GABAergic synaptic activity.7 Magnetic resonance imaging (fMRI) performed at baseline and after NIBS patients show that decreases in GABA are associated with motor recovery in chronic settings as well.1 While the relationship is between GABA and motor learning is apparent, further research is necessary on how total concentrations GABA within larger volumes of cortical tissue relate to the synaptic activity10, and establish which mechanisms decrease GABA and are responsible for neural remapping.8
Identifying Biomarkers
Pairing NIBS with MRS and fMRI addresses many technical problems associated with using the techniques separately. Data are collected at the same point in time, rather separately, increasing reliability and strengthening the relationship between NIBS techniques and changes before, during, and after interventions2. fMRI-MRS techniques are shown to provide information on neural activity and neurochemical concentrations of various brain states, respectively.4 Physiological and cognitive variables are then easier to identify, and support link between the motor cortex and its chemical milieu.6 This comparability is especially important when trying to link hemodynamic and neurochemical responses, the former of which ceases after prolonged periods of stimulation. 4
What does this help us understand?
This research should help inform future rehabilitative techniques that aim to promote neural plasticity and at least partial restoration of motor functioning in post-stroke patients and assist in the stratification of patient treatment and help isolate which behavioral changes and interventions will most benefit any given patient8, in addition to determining the neuro-metabolic mechanisms responsible for learning, regaining motor-functioning, and specific for task encoding.3
References
- Blicher, J., Near, J., Næss-Schmidt, E., Stagg, C., Johansen-Berg, H., & Nielsen, J. et al. (2014). GABA Levels Are Decreased After Stroke and GABA Changes During Rehabilitation Correlate With Motor Improvement. Neurorehabilitation And Neural Repair, 29(3), 278-286. http://dx.doi.org/10.1177/1545968314543652
- Carmichael, S. (2012). Brain Excitability in Stroke. Archives Of Neurology, 69(2), 161. http://dx.doi.org/10.1001/archneurol.2011.1175
- Floyer-Lea, A. (2006). Rapid Modulation of GABA Concentration in Human Sensorimotor Cortex During Motor Learning. Journal Of Neurophysiology, 95(3), 1639-1644. http://dx.doi.org/10.1152/jn.00346.2005
- Ip, I., Berrington, A., Hess, A., Parker, A., Emir, U., & Bridge, H. (2017). Combined fMRI-MRS acquires simultaneous glutamate and BOLD-fMRI signals in the human brain. Neuroimage, 155, 113-119. http://dx.doi.org/10.1016/j.neuroimage.2017.04.030
- Koch, G., Ponzo, V., Di Lorenzo, F., Caltagirone, C., & Veniero, D. (2013). Hebbian and Anti-Hebbian Spike-Timing-Dependent Plasticity of Human Cortico-Cortical Connections. Journal Of Neuroscience, 33(23), 9725-9733. http://dx.doi.org/10.1523/jneurosci.4988-12.2013
- Kolasinski, J., Logan, J., Hinson, E., Manners, D., Divanbeighi Zand, A., & Makin, T. et al. (2017). A Mechanistic Link from GABA to Cortical Architecture and Perception. Current Biology, 27(11), 1685-1691.e3. http://dx.doi.org/10.1016/j.cub.2017.04.055
- Mullins, P., McGonigle, D., O’Gorman, R., Puts, N., Vidyasagar, R., Evans, C., & Edden, R. (2014). Current practice in the use of MEGA-PRESS spectroscopy for the detection of GABA. Neuroimage, 86, 43-52. http://dx.doi.org/10.1016/j.neuroimage.2012.12.004
- Sato, S., Bergmann, T., & Borich, M. (2015). Opportunities for concurrent transcranial magnetic stimulation and electroencephalography to characterize cortical activity in stroke. Frontiers In Human Neuroscience, 9. http://dx.doi.org/10.3389/fnhum.2015.00250
- Stagg, C. (2014). Magnetic Resonance Spectroscopy as a tool to study the role of GABA in motor-cortical plasticity. Neuroimage, 86, 19-27. http://dx.doi.org/10.1016/j.neuroimage.2013.01.009
- Stagg, C., Bestmann, S., Constantinescu, A., Moreno Moreno, L., Allman, C., & Mekle, R. et al. (2011). Relationship between physiological measures of excitability and levels of glutamate and GABA in the human motor cortex. The Journal Of Physiology, 589(23), 5845-5855. http://dx.doi.org/10.1113/jphysiol.2011.216978