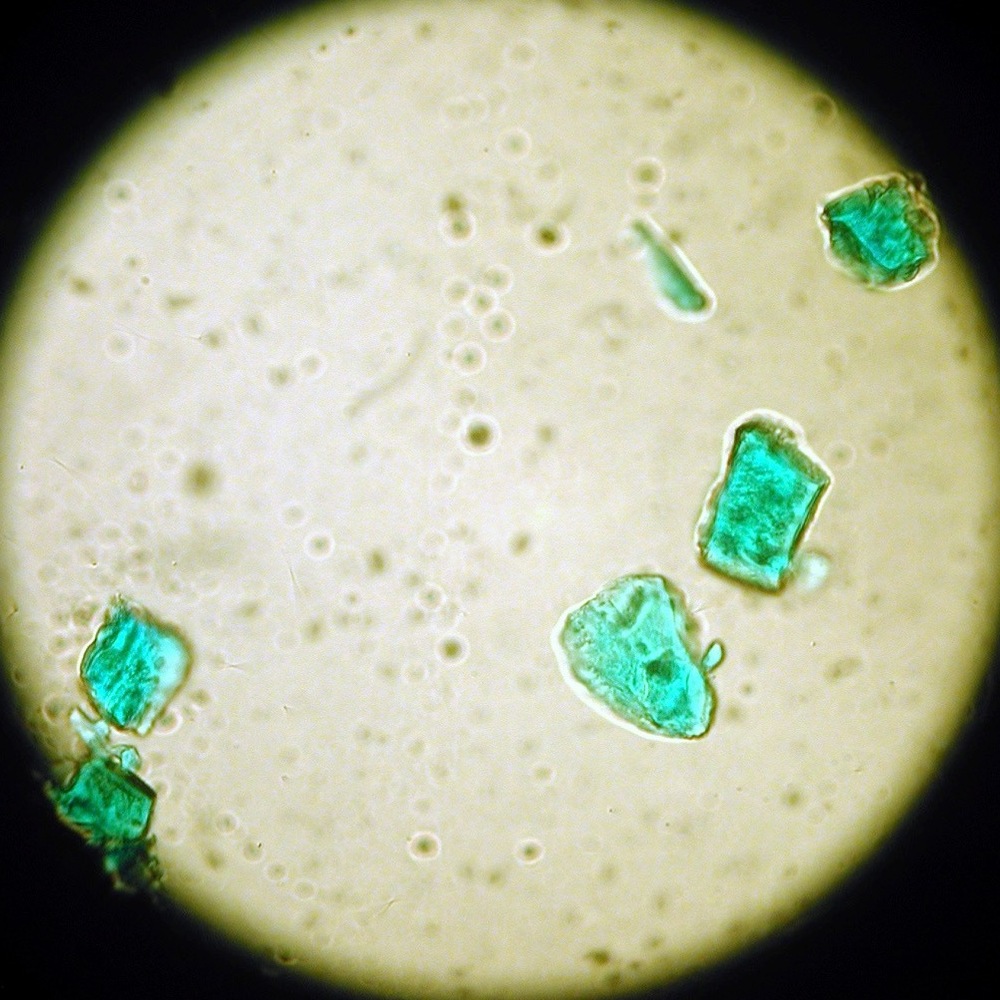
Metal objects corrode due to environmental exposure. This lab activity examines the corrosion of copper by an acidic environment. Students will induce the corrosion and propose the chemical equation for the corrosion product.
Over time, copper and copper alloys corrode in outdoor environments. Outdoor sculpture and architectural details made of copper or copper alloys can develop a patina of corrosion. This patina is sometimes considered attractive and desirable. But corrosion can also be damaging, destroying the surface and eventually the object as well as staining adjacent materials. This colorful mixture of compounds may have several components including copper oxides such as copper acetate. Historically, colored corrosion products have been used as pigments in paint. In this experiment, students will examine the corrosion of copper by an acidic environment. Students will cause the corrosion products to form and propose a balanced chemical equation for the corrosion of copper using acetic acid.
Objectives:
Examine the way copper corrodes
- Write a balanced chemical equation for the formation of copper acetate
S5P2. Students will explain the difference between a physical change and a chemical change.
c. Investigate the properties of a substance before, during, and after a chemical reaction to find evidence of change.
8th grade Science Georgia Performance Standards:
S8P1. Students will examine the scientific view of the nature of matter.
d. Distinguish between physical and chemical properties of matter as physical (i.e., density, melting point, boiling point) or chemical (i.e., reactivity, combustibility).
e. Distinguish between changes in matter as physical (i.e., physical change) or chemical (development of a gas, formation of precipitate, and change in color).
9-12th grade Science Georgia Performance Standards:
SPS2. Students will explore the nature of matter, its classifications, and its system for naming types of matter.
b. Predict formulas for stable binary ionic compounds based on balance of charges.
c. Use IUPAC nomenclature for transition between chemical names and chemical formulas of; binary ionic compounds and binary covalent compounds (containing representative elements, i.e. carbon dioxide)