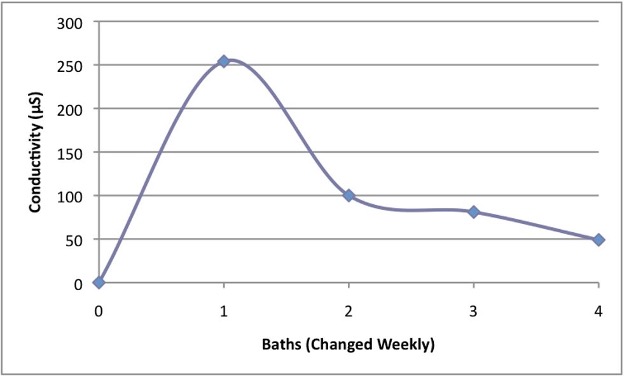
Salt deposits can crystallize within porous materials such as ceramics or stone, causing damage that can destroy the surface and/ or weaken the structure. This lab activity identifies the salts on ceramic fragments using chemical spot tests and also monitors the extraction of soluble salts through successive water baths using conductivity. Salt deposits can crystallize within porous materials such as ceramics. This process causes damage known as flaking or spalling, which can destroy object surfaces. Identifying the salts present in an artifact is vital for determining the course of treatment. If the salts are identified and characterized as soluble, one of the most common treatment procedures is to soak the artifact in distilled water. Conservators monitor the treatment progress by measuring the conductivity of the successive water baths. This experiment uses chemical spot tests to discover the chemical formula of a salty build-‐up on ceramic material. The second half of the experiment investigates conductivity and how the practice of bathing ceramics works.
Objectives:
- Identify the unknown ions present
- Predict ionic compound formulas
- Observe changes in conductivity levels
- Graph conductivity data
Georgia Performance Standards:
SB1. Obtain, evaluate, and communicate information to analyze the nature of the relationships between structures and functions in living cells.
d. Plan and carry out investigations to determine the role of cellular transport (e.g., active, passive, and osmosis) in maintaining homeostasis.
SC1. Obtain, evaluate, and communicate information about the use of the modern atomic theory and periodic law to explain the characteristics of atoms and elements.
a. Evaluate merits and limitations of different models of the atom in relation to relative size, charge, and position of protons, neutrons, and electrons in the atom.
g. Develop and use models, including electron configuration of atoms and ions, to predict an element’s chemical properties.
SC2. Obtain, evaluate, and communicate information about the chemical and physical properties of matter resulting from the ability of atoms to form bonds
c. Construct an explanation about the importance of molecular-level structure in the functioning of designed materials. (Clarification statement: Examples could include why electrically conductive materials are often made of metal, flexible but durable materials are made up of long chained molecules, and pharmaceuticals are designed to interact with specific receptors.)