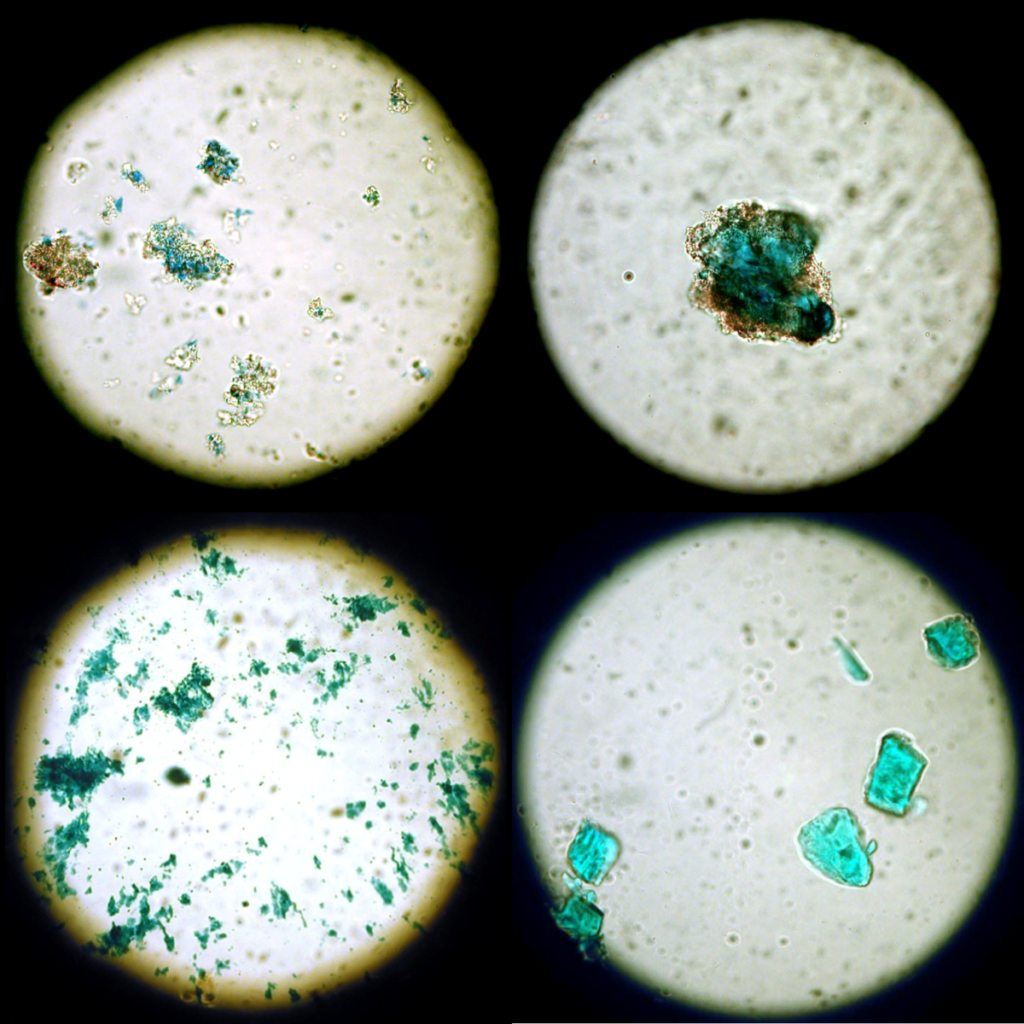
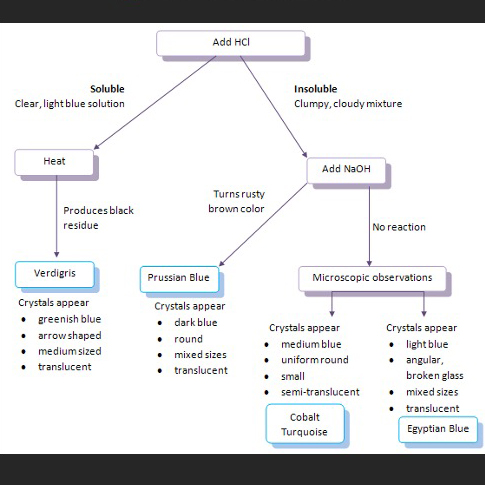
Pigments are inorganic or organic colorants that can be mixed with a binder to form paint. Pigments are typically finely ground particles that have unique physical properties such as color, size, shape, and translucency as well as chemical properties such as solubility, pH, and reactivity to temperature. This lab activity differentiates historic blue pigments using microscopy and chemical spot tests.
Paint consists of a binder, such as oil, resin or gum, mixed with a colorant. Throughout history painters have used a wide variety of organic and inorganic substances to produce color in their work. Inorganic colorants are typically finely ground powders called pigments. In addition to their colors, pigments have unique properties such as particle size, shape, and translucency; solubility; or reactivity to temperature changes and pH. These physical and chemical properties can be examined to distinguish pigments. Since some pigments are characteristic of certain time periods, pigment identification can help with dating and verification. Because pigments react and age differently, identifying the pigments present may influence decisions about the conservation treatment of a painting or painted object. In this activity, students will use microscopy and chemical spot tests to identify a set of blue pigments.
Objectives:
Make qualitative observations of unknown pigments.
Use chemical tests to identify unknown pigments.
Use microscopy to identify unknown pigments.
Georgia Performance Standards:
S8P1. Obtain, evaluate, and communicate information about the structure and properties of matter.
d. Construct an argument based on observational evidence to support the claim that when a change in a substance occurs, it can be classified as either chemical or physical.
SC2. Obtain, evaluate, and communicate information about the chemical and physical properties of matter resulting from the ability of atoms to form bonds.
b. Construct an argument by applying principles of inter- and intra- molecular forces to identify substances based on chemical and physical properties.
SC6. Obtain, evaluate, and communicate information about the properties that describe solutions and the nature of acids and bases.
f. Use mathematics and computational thinking to compare, contrast, and evaluate the nature of acids and bases in terms of percent dissociation, hydronium ion concentration, and pH.