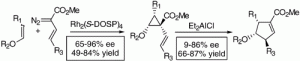Authors: Huw M. L. Davies , Norman Kong , and Melvyn Rowen Churchill
J. Org. Chem.,
1998, 63 (19), 6586–6589
Rhodium N-(dodecylbenzenesulfonyl)prolinate [(Rh2(S-DOSP)4 (1)]-catalyzed decomposition of diazobutenoates in the presence of vinyl ethers results in highly diastereoselective and enantioselective synthesis of donor/acceptor-substituted vinylcyclopropanes. Diethylaluminum chloride-induced rearrangement of these vinylcyclopropanes results in the formation of cyclopentenes with excellent control of diastereoselectivity and for the fused cyclopentenes good control of absolute stereochemistry. This study illustrates the synthetic utility of Rh2(S-DOSP)4 as a chiral catalyst for vinylcarbenoid cyclopropanations and the remarkable diastereocontrol that is possible in the diethylaluminum chloride-induced ring expansion of donor/acceptor-substituted vinylcyclopropanes.