Authors: Huw M. L. Davies , Pingda Ren , and Qihui Jin
Org. Lett.,
2001, 3 (22), 3587–3590
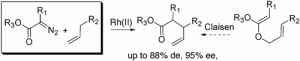
Tetrakis[N-[4-dodecylphenyl)sulfonyl]-(S)-prolinato]-dirhodium [Rh2(S-DOSP)4] catalyzed decomposition of methyl aryldiazoacetates in the presence of alkenes results in allylic C−H activation by means of a rhodium-carbene induced C−H insertion. The resulting γ,δ-unsaturated esters are equivalent to products that would be traditionally obtained from an asymmetric Claisen rearrangement. Highly regio- and enantioselective C−H insertions can be achieved, and in certain cases, good diastereocontrol is also possible.