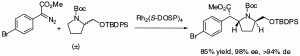Authors: Huw M. L. Davies, Chandrasekar Venkataramani, Tore Hansen and Darrin W. Hopper
J. Am. Chem. Soc.,
2003, 125 (21), 6462–6468
The asymmetric C−H activation reactions of methyl aryldiazoacetates are readily induced by the rhodium prolinate catalyst Rh2(S-DOSP)4 (1) or the bridged prolinate catalysts Rh2(S-biDOSP)2 (2a) and Rh2(S-biTISP)2 (2b). The C−H activation of N-Boc-protected cyclic amines demonstrates that the donor/acceptor-substituted carbenoids display remarkable chemoselectivity, which allows for highly regioselective, diastereoselective, and enantioselective reactions to be achieved. Furthermore, the reactions can display high levels of double stereodifferentiation and kinetic resolution. The C−H activation is caused by a rhodium carbenoid induced C−H insertion. The potential of this chemistry is demonstrated by a very direct synthesis of threo-methylphenidate.