Authors: Huw M. L. Davies, Xing Dai , and Matthew S. Long
J. Am. Chem. Soc.,
2006, 128 (7), 2485–2490
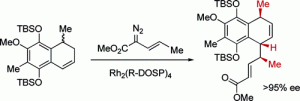
The total synthesis of (−)-colombiasin A (2) and (−)-elisapterosin B (3) has been achieved. The key step is a C−H functionalization process, the combined C−H activation/Cope rearrangement, between methyl (E)-2-diazo-3-pentenoate and 1-methyl-1,2-dihydronaphthalenes. When the reaction is catalyzed by dirhodium tetrakis((R)-(N-dodecylbenzenesulfonyl)prolinate), Rh2(R-DOSP)4, an enantiomer differentiation step occurs where one enantiomer of the dihydronaphthalene undergoes the combined C−H activation/Cope rearrangement while the other undergoes cyclopropanation. This sequence controls the three key stereocenters in the natural products such that the remainder of the synthesis is feasible using standard chemistry.