Authors: Xing Dai , Zhongliang Wan , Russell G. Kerr and Huw M. L. Davies
J. Org. Chem.,
2007, 72 (6), 1895–1900
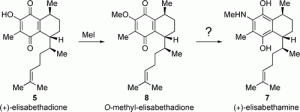
These studies were conducted to determine the discrepancies between the spectroscopic data of the isolated and synthetic samples of the marine natural product (+)-elisabethadione. Attempts at the re-isolation of (+)-elisabethadione from Pseudopterogorgia elisabethae were unsuccessful, but during these efforts, two related natural products of O-methylelisabethadione (8) and O-methyl-nor-elisabethadione (9) were discovered. The total syntheses of these new natural products were accomplished by using the combined C−H activation/Cope rearrangement as the key step and the previously synthesized elisabethadione was converted to O-methylelisabethadione. These studies confirmed that the synthetic sample of (+)-elisabthadione was assigned the correct structure. The considerable differences in the data between the synthetic and natural samples of (+)-elisabethadione lead to the conclusion that the structure of the natural material was either miss-assigned or the published spectral data were incorrect. During the course of these studies, questions arose about the assigned structure of another natural product, elisabethamine, which was proposed to be an aminohydroquinone. Attempts at the synthesis of this compound revealed that the aminohydroquinone structure was unstable in air as it was readily oxidized to the quinone.