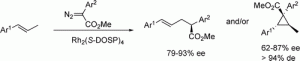Authors: Huw M. L. Davies , Michael G. Coleman , and Dominic L. Ventura
Org. Lett.,
2007, 9 (24), 4971–4974
Rhodium(II)-catalyzed reactions of aryldiazoacetates with (E)-aryl-substituted alkenes generate C−H insertion products and/or cyclopropanes. The product distribution is influenced by the nature of the donor group on the carbenoid, the structure of the (E)-aryl-substituted alkenes, and the rhodium catalyst.