Authors: Changming Qin, Vyacheslav Boyarskikh, Jørn H. Hansen, Kenneth I. Hardcastle, Djamaladdin G. Musaev, and Huw M. L. Davies
J. Am. Chem. Soc.,
2011, 133 (47), 19198–19204
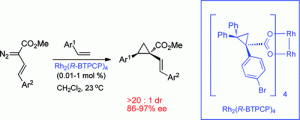
Dirhodium tetrakis-(R)-(1-(4-bromophenyl)-2,2-diphenylcyclopropanecarboxylate) (Rh2(R-BTPCP)4) was found to be an effective chiral catalyst for enantioselective reactions of aryl- and styryldiazoacetates. Highly enantioselective cyclopropanations, tandem cyclopropanation/Cope rearrangements and a combined C–H functionalization/Cope rearrangement were achieved using Rh2(R-BTPCP)4 as catalyst. The advantages of Rh2(R-BTPCP)4 include its ease of synthesis, its tolerance to the size of the ester group in the styryldiazoacetates, and its compatibility with dichloromethane as solvent. Computational studies suggest that the catalyst adopts a D2-symmetric arrangement, but when the carbenoid binds to the catalyst, two of the p-bromophenyl groups on the ligands rotate outward to make room for the carbenoid and the approach of the substrate to the carbenoid.