Prof. Brian Dyer’s research group at Emory is currently interested in interactions of components of the hemagluttinin protein of the influenza virus with lipid bilayers and with each other in the lipid bilayer environment. We are collaborating with graduate student Alexia Prokopik and recent Ph.D. Samuel Jeong from the Dyer lab on using molecular dynamics methods to complement their experimental plans. More to come!
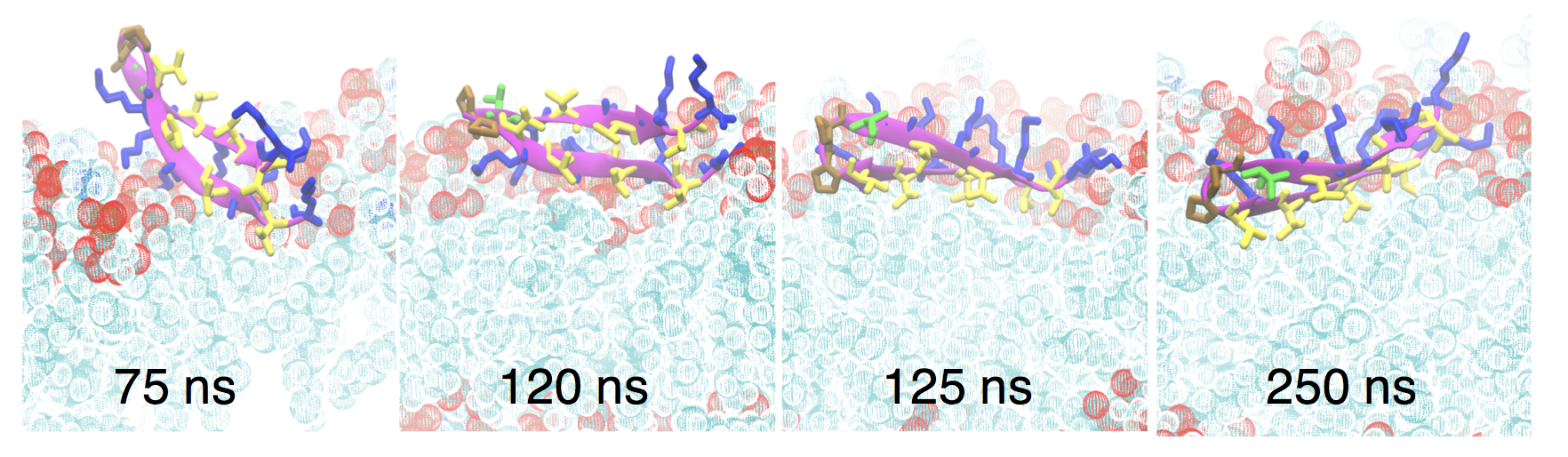
Ph.D. student Keon Reid has been using molecular dynamics simulation to understand the behavior of the anti-cancer/anti-microbial peptide SVS-1 in collaboration with members of the Dyer group. A manuscript describing Keon’s simulations and experiments by Dr. Caitlin Davis on this peptide has recently been accepted for publication:
K. A. Reid, C. M. Davis, R. B. Dyer, and J. T. Kindt, “Binding, folding, and insertion of a beta-hairpin peptide at a lipid bilayer surface: Influence of electrostatics and lipid tail packing.” Biochim. Biophys Acta – Biomembranes link
Simulation snapshots showing “flip and dip” mechanism for SVS-1 to move from an on-top position to a partially inserted state in a POPG/POPC mixed lipid bilayer under applied surface tension.