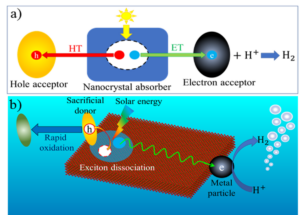
Converting solar energy to chemical fuels through artificial photosynthesis addresses both solar energy conversion and storage problems. Quantum confined semiconductor nanocrystals have emerged as promising light absorption and charge separation materials because they have large absorption cross sections and their band gap, band edge positions and charge separation properties can be tuned through size, shape and composition. They have been integrated with metal particles, molecular catalysts or enzymes to form semiconductor-catalyst nano-heterostructures that have demonstrated promising performances for light-driven fuel formation reactions, such as H2 generation, CO2 reduction and N2 fixation. Despite these demonstrations, many fundamental challenges remain. The rational design and improvement of these photosynthetic systems requires the understanding of the mechanisms of long-distance charge separation and recombination in these nano-heterostructures. Furthermore, many catalytic reactions require that multiple proton coupled electron transfer (PCET) to the catalytic active site to avoid energetic intermediates. It remains unclear how to design colloidal nanoparticle/catalyst complexes to enhance PCET activities.
- a) Scheme of artificial solar-to-H2 conversion systems based on low-dimensional colloidal nanocrystals. HT and ET: hole and electron transfer, respectively. (b) Exciton dissociation and light-driven H2 generation in nanoplatelet-metal heterostructures.
Current ongoing projects of this DOE funded program are focused on understanding
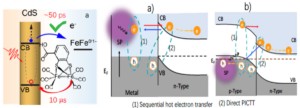
a) multiple electron transfer from quantum-confined semiconductor nanocrystals to adsorbed molecule catalysts for efficient light driven proton or CO2 reduction,
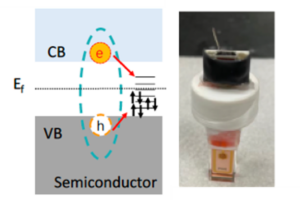
b) the effect of external bias on charge separation and recombination in semiconductor/metal heterostructures in solution,
c) plasmon induced hot electron transfer from plasmonic nanoparticles.