|
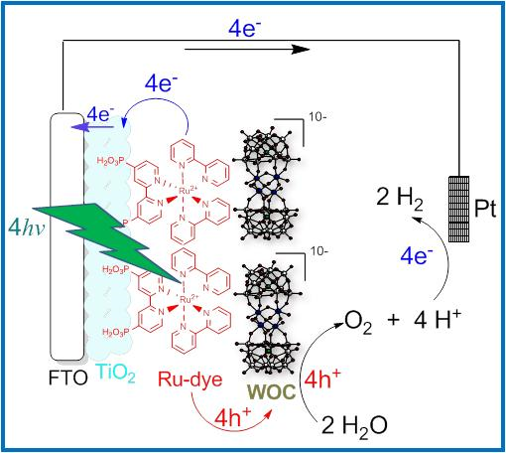
Direct conversion of solar energy into clean fuel is one of the most important scientific challenges in solar energy conversion. In this project, Professors Craig Hill, Tianquan Lian and Djamaladdin Musaev at Emory University are developing robust and carbon-free nano-scaled assemblies that are capable of converting solar energy into fuel by splitting water. The overall thrust of this renewal application is to conduct fundamental research on the success limiting factors in realizing more efficient and stable visible-light driven water oxidation/splitting systems. Central to this 3-team effort is a closely integrated experimental and computational research program targeting the synthetic, photophysical and photochemical properties of photo-driven water oxidation dyads and triads containing semiconductor metal oxides (SMOs) associated with soluble (molecular), stable, and fast water oxidation catalysts (WOCs) based on polyoxometalates (POMs). The synthesis, structural characterization, photoelectrochemical performance (O2 yield and water oxidation photocurrent) and interfacial charge transfer processes of the dyads and triads will be examined by integrated experimental and computational studies. The proposed research will provide important scientific insight into how to design and optimize catalysts, inorganic photosensitizers, and their nanoassemblies for photocatalytic water oxidation. Major accomplishments of this research program should strongly impact future solar photochemistry research via design and development of (a) new and more ambitious experimental and computational methodology, (b) more efficient and stable molecular WOCs, (c) more robust all-inorganic molecular chromophores, and (d) more stable and efficient POM WOC-based dyadic and triadic photoanodes for solar driven water oxidation. We acknowledge generous financial support by DOE |
|