Author: Demetrice S. Bryant
-
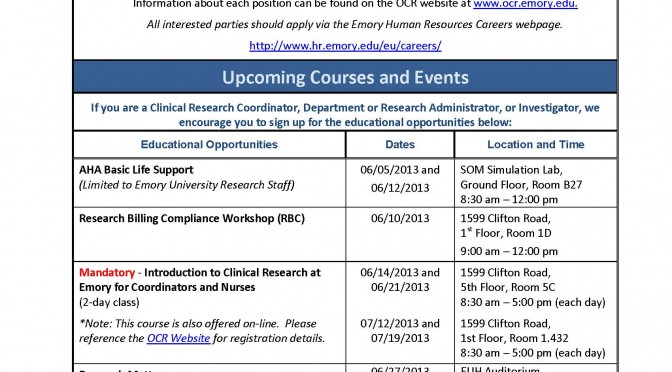
OCR Professional Development Opportunities
The Office for Clinical Research is pleased to announce its professional development opportunities, as well as three new job opportunities in the central office. Please download the attached flyer for more information at: June 2013 Newsletter Final or click the image below to review in the browser.
-
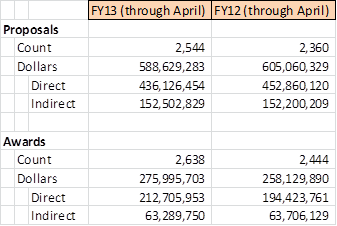
OSP Statistics: Proposals Submitted and Awards Received
Available below are our counts through April, FY13 and April, FY12 for proposals submitted by Emory University and Awards received by Emory University. Please note that variances between the years can be impacted by many variables and can change significantly between now and the end of the fiscal year (August). For further details or information,…
-
OGCA’s New Help Desk
Through the exceptional work of our faculty, Emory’s research funding and activity has grown significantly over the past decade. In an effort to provide better post-award support, OGCA is implementing help desks. Help desk services will be fully operational by May 15, 2013. To promote personal service, each department will be assigned to a team.…
-
New OSP & OGCA Websites are Coming!
Effective June 1, 2013, many of the ORA offices will have new websites including OSP and OGCA. There will be no change to the URL’s that you currently utilize to access our websites. If you have any questions, please contact Demetrice Bryant at dbryant@emory.edu or 404-727-5581. Demetrice Bryant Director, Education & Communications OSP & OGCA
-
Process For Review of Confidentiality/ Non-Disclosure Agreements
In an effort to improve efficiency and the handling of confidential/non-disclosure agreements (CDAs/NDAs), the OSP Contracts Unit has established a listserv e-mail address to send CDAs requiring review and institutional signature. Effective February 1, 2012, all clinical trial related CDAs/NDAs requiring review and institutional signature should be sent to the listserv e-mail. The listserv e-mail…
-
Getting Grants Access
Often times we receive questions regarding grant access for PI’s. More specifically, “Do I need to route a PI Eligible Form for my PI to have grants access? Grants access and PI eligibility are not automatically provided at the time that a faculty member’s Net ID is provided from the University. This access must be…
-
F & A Reminder
The Facilities and Administrative (F&A) rate is a percentage measure of the overhead (indirect) costs incurred to support sponsored activity (including organized research, other sponsored activities, and sponsored instruction) conducted by the University. The application of the rate is the mechanism used to reimburse the University for overhead costs incurred in supporting these activities. At…
-
Reporting Update: Sponsored Project Trend Report Available in FORS
The Sponsored Project Trend report was recently taken offline due to technical issues with the report. These issues have since been resolved and the report is again available in FORS. If you are not familiar with this report, it shows expenses for a project across a 12-month period, allowing any changes in trends to be…
-
NIH Public Access Policy Reminders
As NIH moves toward enhanced enforcement of their Public Access Policy, please bear in mind these important reminders. Applicability The policy applies to all peer-reviewed articles that arise, in whole or in part, from direct costs funded by NIH that are accepted for publication on or after April 7, 2008. Publications that are accepted for…
-
NIH Transition to Research Performance Progress Report (RPPR)
The National Institutes of Health has announced that use of the Research Performance Progress Report (RPPR) will become mandatory for awards under the Streamlined Noncompeting Award Process (SNAP) and Fellowship Awards (F series) which have budget start dates on or after July 1, 2013. (SNAP continuation proposals for these awards are due no later than…