Category: OCR
-
OCR Professional Development Opportunities
The Office for Clinical Research is pleased to announce its professional development opportunities, as well as three new job opportunities in the central office. Please download the attached flyer for more information at: August 2013 Newsletter or click the image below to review in the browser.
-
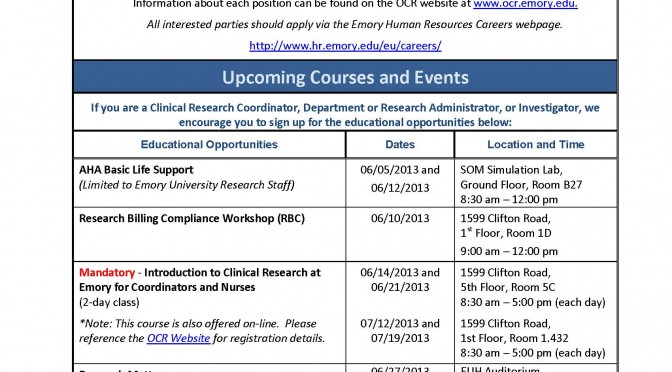
OCR Professional Development Opportunities
The Office for Clinical Research is pleased to announce its professional development opportunities, as well as three new job opportunities in the central office. Please download the attached flyer for more information at: June 2013 Newsletter Final or click the image below to review in the browser.
-
From the Office for Clinical Research (OCR)
From the Office for Clinical Research (OCR) Announcements Upcoming Courses and Events If you are a Clinical Research Coordinator, Department or Research Administrator, or Principal Investigator, we encourage you to sign up for the educational opportunities below: Educational Opportunities Dates Location and Time AHA Basic Life Support (Limited to Emory University Research Staff) 04/03/2013 or …
-
From the Office for Clinical Research (OCR)
From the Office for Clinical Research (OCR) Announcement The deadline date for the mandatory training requirements set forth by the School of Medicine, the Clinical Trials Operations Committee, and the Clinical Trials Executive Committee is March 7, 2013. This training applies to all clinical research coordinators, nurses, residents and fellows participating as key personnel in…
-
From the Office of Clinical Research (OCR)
From the Office for Clinical Research (OCR) Announcement OCR’s Research Matters educational event held on October 25, 2012 is available online! View the recorded event via the Emory Learning Management System (eLMS). From “My Learning” page, click Browse Catalog > Clinical Research Education > Research Matters > select Recorded Presentations: Research Matters (205036), and enroll…
-
From the Office of Clinical Research
In an effort to streamline the budget development process and improve turn-around times, OCR will pilot the development of limited budgets for all non-negotiable budgets. OCR will continue to create a PRA for all studies if there are EHC billables. However, OCR will create the ERMS budget only for those visits with EHC billables that have CPT codes to be charged…
-
From the Office for Clinical Research (OCR)
From the Office for Clinical Research (OCR) Announcements There are new training requirements set forth by the School of Medicine, the Clinical Trials Operations Committee, and the Clinical Trials Executive Committee. This training applies to all clinical research coordinators and research nurses participating as key personnel in clinical trials. The course is designed…
-
From The Office for Clinical Research (OCR)
Announcements The Clinical Trials Audit and Compliance (CTAC) Office has established a new university website at www. ctac.emory.edu. CTAC auditors will be visiting study locations in the upcoming months to provide education on the web- site and Clinical Trials Guidebook. To schedule a site review, please contact Stephanie deRijke at smickle@ emory.edu. Helpful Tips When…
-
From the Office of Clinical Research (OCR)
From the Office for Clinical Research (OCR) ERMS Quick Reference Guide ERMS Visit Tracking, a new feature within the Emory Research Management System (ERMS) has officially replaced SiteMinder effective Monday, November 14, 2011. The new feature will be a huge improvement to the efficiency of your process, by significantly increasing billing…