Based on their low concentrations in mammalian brain, octopamine, β–phenylethylamine, tyramine, and tryptamine are classified as “trace” amines (TAs) and viewed as metabolic by-products. TAs are related to the classical monoamine transmitters and are synthesized from the same precursor aromatic amino acids. The recent discovery of a family of G protein-coupled receptors preferentially activated by TAs rekindled interest in TAs, but without a known circuitry, their function remains elusive and understudied. We now have anatomical evidence of an endogenous TA system in spinal cord, and show that the TAs recruit locomotor circuits and modulate ongoing locomotion. Our newest evidence suggest the TAs represent a parallel biochemical modulatory system that alters circuit function via a membrane transporter shuttling system that operates independent of synaptic actions. The long-term goal is to understand the physiological relevance of the TAs as an intrinsic modulatory system for subsequent therapeutic manipulation of spinal circuit function.
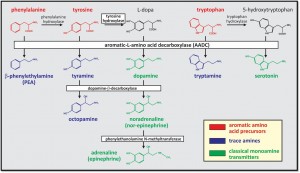
Comparison of monoamine synthesis pathways. The trace amines (TAs) are a group of endogenous monoamines that include tryptamine, tyramine, octopamine and β-phenylethylamine (PEA; blue). The TAs have structural, metabolic, physiologic, and pharmacologic similarities to the classical monoamine transmitters (green) and are synthesized from the same precursor aromatic amino acids (red). Unlike the classical monoamines, aromatic-L-amino acid decarboxylase (AADC; also called dopa decarboxylase) is the only enzyme required to produce them. Conversion from the TAs to the monoamines does not appear to occur.
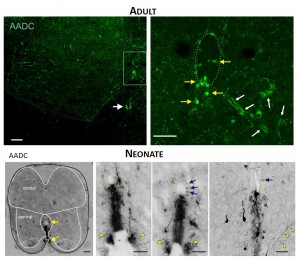
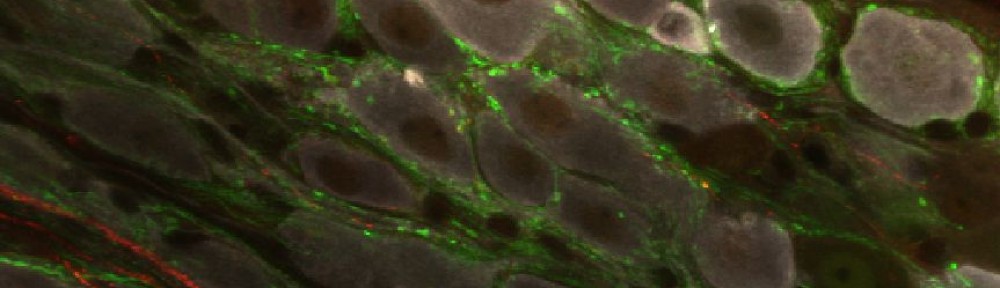
TOP. LEFT. Intense AADC immunolabeling is found in blood vessels, in the D cells and in the ventromedial white matter (white arrow in left panel). Note that some AADC+ neurons are also found in the dorsal central canal. RIGHT. Higher power image of central canal region (outlined). Blood vessels are identified with white arrows and AADC+ neurons with yellow arrows. BOTTOM. LEFT. Low power transverse spinal cord section (10 µm) of AADC immunolabeling. Superimposed is an outline of the spinal cord (black) with interior white lines approximately identifying dorsal and ventral gray matter and central canal region. Note strongest labeling is associated with D cells intermingled with epithelial cells surrounding the central canal (top arrow). Also, note the associated vertical row of cellular labeling projecting ventrally, and bilateral white matter labeling in the ventral funiculus (bottom arrow). RIGHT. Three panels showing higher power images from separate sections illustrate the diversity of AADC labeling in the ventral medial grey matter region. Epithelial cell layer surrounding central canal is outlined while blue arrows point to example D cells. Common to all is the vertical stream of projections with intermingled cells ventral to the central canal. These appear to end in a white matter tract in the ventral funiculus (yellow arrowheads).
-
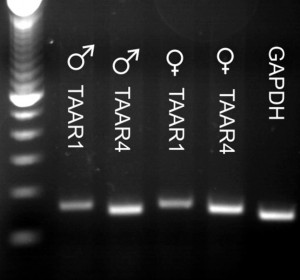
TAARs are expressed in neonatal rat spinal cord. RT-PCR evidence of TAAR expression in both sexes. In males, TAARs 2,5, and 6 expression was weakest, and in females, TAARs 2,6, and 9 were weakest. RT-PCR series directly comparing TAAR1 and TAAR4 expression in both sexes as these receptors are known to be activated by trace amines.
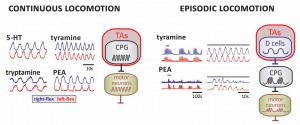
LEFT. Examples of continuous locomotor rhythms generated in the presence of 5-HT and the TAs. Shown are smoothed activity pattern envelopes reporting activity for right (blue) and left flexors (red). RIGHT. Episodic bouts of locomotor-like rhythms are shown for tyramine and PEA at slow (left) and expanded time scales (right). Bar over epochs at left identified expanded waveform at right. Proposed circuit locations for TA-induced modulatory actions in the emergence of continuous and episodic locomotor rhythms. TA-induced slower activity rhythms on neurons that drive the CPG could lead to the episodic waxing and waning of CPG output to motor neurons. Candidate neurons are the lamina X AADC+ D cells.
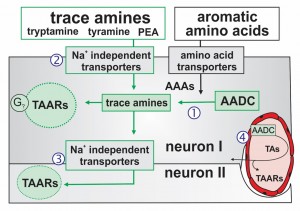
Putative transport and intracellular signaling mechanisms for observed TA actions. The TAs are synthesized from their precursor aromatic amino acids (AAAs) via the essential synthesis enzyme AADC [1]. Intracellular TAs act on TAARs to produce G protein-coupled neuromodulatory responses. TA transport into neurons via Na+-independent membrane transporters allows for TAAR-containing neurons to also be modulated by TA following their exogenous application‚[2], (ii) release from AADC expressing neurons [3]; here neuron I onto neuron II) or (iii) release from AADC-expressing endothelia [4].
Gozal, E.A., O’Neill, B., Sawchuk, M.A., Zhu, H., Halder, M., Chou C-C., and Hochman, S. Anatomical and functional evidence for trace amines as unique modulators of locomotor function in the mammalian spinal cord. Front Neural Circuits. (2014) Front. Neural Circuits, 07 November 2014 | doi: 10.3389/fncir.2014.00134.
HOCHMAN,S. Metabolic recruitment of spinal locomotion: intracellular neuromodulation by trace amines and their receptors. Neural Regeneration Research. In press (2015)