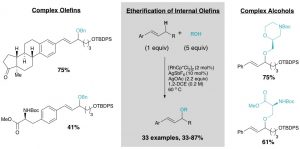
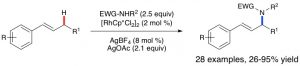
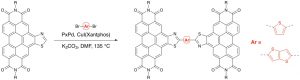
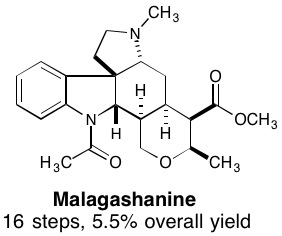
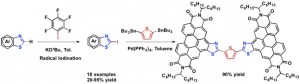
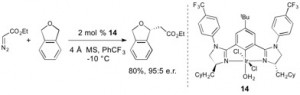
29. Intermolecular Allylic C-H Etherification of Internal Olefins
Taylor A. F. Nelson, Simon B. Blakey Angew. Chem. Int. Ed. 2018, DOI:10.1002/anie.201809863
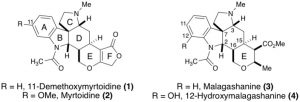
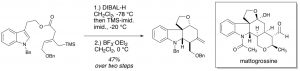
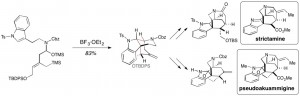
28. Model Studies for the Total Synthesis of 11-Demethoxymyrtoidine and Myrtoidine
Amaan M. Kazerouni, Danny E. Mancheno, Simon B. Blakey Heterocycles (Special Issue Honoring Tohru Fukuyama). 2018
Qinqin Shi, Wesley Tatum, Junxiang Zhang, Colleen Scott, T, Christine K. Luscombe, Seth R. Marder, Simon B. Blakey Asian J. Org. Chem. 2018, 7, 1419; DOI:10.3390/molecules22030922
Junxiang Zhang, Lauren J. Kang, Timothy C. Parker, Simon B. Blakey, Christine K. Luscombe, Seth R. Marder Molecules 2018, 23, 922; DOI:10.1002/ajoc.201800232
Jacob Burman, Simon B. Blakey, Angew. Chem. Int. Ed. 2017, 82, 10139; DOI:10.1002/anie.201707021
Siyuan Zhang, Junxiang Zhang, Maged Abdelsamie, Qinqin Shi, Yadong Zhang, Timothy C. Parker, Evgheni V. Jucov, Tatiana V. Timofeeva, Aram Amassian, Guillermo C. Bazan, Simon B. Blakey, Stephen Barlow, and Seth R. Marder Chem. Mater. 2017, 29, 7880; DOI:10.1021/acs.chemmater.7b02665
Qinqin Shi, Eric S. Andreansky, Seth R. Marder, Simon B. Blakey, J. Org. Chem. 2017, 82, 10139; DOI:10.1021/acs.joc.7b01604
Aidi Kong, Eric S. Andreansky, Simon B. Blakey, J. Org. Chem. 2017, 82, 4477; DOI:10.1021/acs.joc.7b00503
Eric S. Andreansky, Simon B. Blakey, Org. Lett. 2016, 18, 6492. DOI: 10.1021/acs.orglett.6b03406
Aidi Kong, Danny E. Mancheno, Nadege, Boudet, Ricardo Delgado, Eric S. Andreansky, Simon B. Blakey, Chem. Sci. 2017, 8, 697 DOI: 10.1039/c6sc03578g
Highlighted in Synfacts, 2016, 12(12), 1229, DOI: 10.1055/s-0036-1589453
Qinqin Shi, Siyuan Zhang, Junxiang Zhang, Victoria F. Oswald, Aram Amassian, Seth R. Marder, Simon B. Blakey, J. Am. Chem. Soc. 2016, DOI: 10.1021/jacs.5b12259
Highlighted in Synfacts, 2016, 12(06), 0587, DOI: 10.1055/s-0035-1562147
Mace Weldy, N. Schafer, A. G.; Owens, C. P.; Herting, C. J.; Varela-Alvarez, A.; Chen, S.; Niemeyer, Z.; Musaev, D. J.; Sigman, M. S.; Davies, H. M. L.; Blakey, S. B. Chem. Sci. 2016, 7, 3142 DOI 10.1039/C6SC00190D
Highlighted in Synfacts, 2016, 12(08), 0817, DOI: 10.1055/s-0035-1562660
17. Cobalt Catalyzed sp3 C–H Amination Utilizing Aryl Azides
Villanueva, O.; Mace Weldy, N.; Blakey, S. B.; MacBeth, C. E. Chem. Sci. 2015, DOI: 10.1039/C5SC01162K
16. Ir-Catalyzed Enantioselective Group Transfer Reactions
Schafer, A. G.; Blakey, S. B. Chem. Soc. Rev. 2015, DOI: 10.1039/C5CS00354G
15. Expanding the Carbene C–H Insertion Toolbox
Morton, D.; Blakey, S. B. Chem. Cat. Chem. 2015, 7, 577
14. A C-H Functionalization Protocol for the Direct Synthesis of Benzobisthiazole Derivatives
Bon, J. L.; Feng, D.; Marder, S. R.; Blakey, S. B. J. Org. Chem. 2014, 79, 7766, DOI: 10.1021/jo501416j
13. Iridium(III)-bis(oxazolinyl)phenyl Catalysts for Enantioselective C-H Functionalization
Owens C. P.; Varela-Álvarez, A.; Boyarskikh, V.; Musaev, D. G.; Davies, . M. L.; Blakey S. B. Chem. Sci. 2013, 4, 2590
Mace, N.; Thornton, A. R.; Blakey, S. B. Angew. Chem. Int. Ed. 2013, 52, 5836
11. Insight into Mechanistic Features of Ruthenium(II)-Pybox-Catalyzed C–H Amination
Musaev, D. G.; Blakey, S. B. Organometallics 2012, 31, 4950
10. Intramolecular Olefin Diamination for the Stereoselective Synthesis of 3-Aminopiperidines
Kong, A.; Blakey, S. B. Synthesis 2012, 44, 1190 (Invited contribution for special issue on hypervalent iodine oxidants)
9. Synthesis of Ruthenium(II) 2,6-Bis(imino)pyridyl Complexes for C-H Amination of Sulfamate Esters
Bon, J. L.; Blakey, S. B.; Heterocycles 2012, 84, 1313 (Invited contribution honoring Prof. Padwa on his 75th birthday)
Stoll, A. H.; Blakey, S. B. Chem. Sci. 2011, 2, 112
7. Copper-Catalyzed Olefin Aminoacetoxylation
Mancheno, D. E.; Thornton, A. R.; Stoll, A. H.; Kong, A.; Blakey, S. B. Org. Lett. 2010, 13, 4110
Stoll, A. H.; Blakey, S. B. J. Am. Chem. Soc. 2010, 132, 2108 (Featured on the cover of issue 7, Feb. 24th 2010)
Delgado, R.; Blakey, S. B. Eur. J. Org. Chem. 2009, 1506
Thornton, A. R.; Martin, V. I.; Blakey, S. B. J. Am. Chem. Soc. 2009, 131, 2434
3. Synthesis and Reactivity of an Unprecedented Os(VIII) Alkylidene
Martin, V. I.; Blakey, S. B. Tetrahedron Lett., 2008, 49, 6800.
2. Enantioselective C–H Amination Using Cationic Ru(II)-pybox Catalysts
Milczek E.; Boudét, N.; Blakey, S. Angew. Chem., Int. Ed., 2008, 47, 6825. (Paper highlighted in Synfacts, 2008, 11, 1184)

Thornton, A. R.; Blakey, S. B. J. Am. Chem. Soc., 2008, 130, 5020. (Paper highlighted in Synfacts, 2008, 7, 691)
Book Chapters
C–N Bond Formation by C–H Functionalization via Metal-Catalyzed Nitrene Insertion
Mace Weldy, N.; Blakey, S. B in “Science of Synthesis”, Wiley VCH, 2015 In press
Enantioselective C-H Amination
Boudét, N.; Blakey, S. In “Chiral Amine Synthesis, Methods, Developments and Applications”, T. C. Nugent Ed.; Wiley-VCH: Weinheim, 2010, p 377-396
Publications Prior to Emory
Total Synthesis of Aplyronine C
Paterson, I.; Fink, S. J.; Lee, L. Y. W.; Atkinson, S. J.; Blakey, S. B. Org. Lett. 2013, 15, 3118
Total Synthesis of Diazonamide A
Knowles, R. R.; Carpenter, J.; Blakey, S. B.; Kayano, A.; Mangion, I. K.; Sinz, C. J.; MacMillan, D. W. C. M. Chem. Sci. 2011, 2, 308
The First Suzuki Cross-Couplings of Aryltrimethylammonium Salts
Blakey, S. B.; MacMillan, D. W. C. J. Am. Chem. Soc. 2003, 125, 6046
Studies in marine macrolide synthesis: stereocontrolled synthesis of a C21-C34 subunit of the aplyronines
Paterson, I.; Blakey, S. B.; Cowden, C. J. Tetrahedron Lett. 2002, 43, 6005
cis-1,2-Dihydrocatechols in Chemical Synthesis: First synthesis of L-ascorbic acid (vitamin C) from a non-carbohydrate source
Banwell, M.; Blakey, S.; Harfoot, G.; Longmore, R. Aust. J. Chem. 1999, 52, 137
First synthesis of L-ascorbic acid (vitamin C) from a non-carbohydrate source
Banwell, M.; Blakey, S.; Harfoot, G.; Longmore, R. J. Chem. Soc., Perkin Trans. 1, 1998, 3141.