Authors: Huw M. L. Davies , Tadamichi Nagashima , and James L. Klino III
Org. Lett.,
2000, 2 (6), 823–826
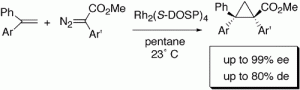
Dirhodium tetrakis(S-(N-dodecylbenzenesulfonyl)prolinate) (Rh2(S-DOSP)4)-catalyzed decomposition of methyl phenyldiazoacetate in the presence of 1,1-diarylethylenes results in intermolecular cyclopropanation with high enantioselectivity (up to 99% ee) and moderate diastereoselectivity (up to 80% de). The reaction was applied to the asymmetric synthesis of a cyclopropyl analogue of tamoxifen.