Authors: Huw M. L. Davies , Rebecca L. Calvo , Robert J. Townsend , Pingda Ren , and R. Melvyn Churchill
J. Org. Chem.,
2000, 65 (14), 4261–4268
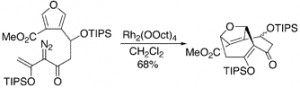
The intramolecular type II [3 + 4] cycloaddition between vinylcarbenoids and furans is a practical method for the construction of 5-oxo-10-oxatricyclo[6.2.1.04,9]undeca-3,8(11)-dienes, containing two anti-Bredt double bonds. These tricyclic systems are well functionalized for eventual elaboration to the natural product CP-263,114. The rhodium-stabilized vinylcarbenoids are generated by dirhodium tetracarboxylate catalyzed decomposition of vinyldiazoacetates. The [3 + 4] cycloaddition is generally considered to occur by a tandem cyclopropanation/Cope rearrangement, although evidence is presented that with these substrates the [3 + 4] cycloaddition may occur in a concerted manner.