Authors: Zhanjie Li , Brendan T. Parr , and Huw M. L. Davies
J. Am. Chem. Soc.,
2012, 134 (26), 10942–10946
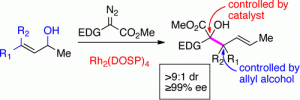
The tandem ylide formation/[2,3]-sigmatropic rearrangement between donor/acceptor rhodium carbenoids and chiral allyl alcohols is a convergent C–C bond forming process, which generates two vicinal stereogenic centers. Any of the four possible stereoisomers can be selectively synthesized by appropriate combination of the chiral catalyst Rh2(DOSP)4 and the chiral alcohol.