Authors: Huw M. L. Davies and Yajing Lian
Acc. Chem. Res.,
2012, 45 (6), 923–935
The development of methods for the stereoselective functionalization of sp3 C–H bonds is a challenging undertaking. This Account describes the scope of the combined C–H functionalization/Cope rearrangement (CHCR), a reaction that occurs between rhodium-stabilized vinylcarbenoids and substrates containing allylic C–H bonds. Computational studies have shown that the CHCR reaction is initiated by a hydride transfer to the carbenoid from an allyl site on the substrate, which is then rapidly followed by C–C bond formation between the developing rhodium-bound allyl anion and the allyl cation. In principle, the reaction can proceed through four distinct orientations of the vinylcarbenoid and the approaching substrate. The early examples of the CHCR reaction were all highly diastereoselective, consistent with a reaction proceeding via a chair transition state with the vinylcarbenoid adopting an s-cis conformation. Recent computational studies have revealed that other transition state orientations are energetically accessible, and these results have guided the development of highly stereoselective CHCR reactions that proceed through a boat transition state with the vinylcarbenoid in an s-cis configuration.
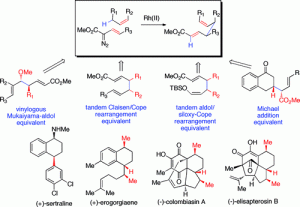
The CHCR reaction has broad applications in organic synthesis. In some new protocols, the CHCR reaction acts as a surrogate to some of the classic synthetic strategies in organic chemistry. The CHCR reaction has served as a synthetic equivalent of the Michael reaction, the vinylogous Mukaiyama aldol reaction, the tandem Claisen rearrangement/Cope rearrangement, and the tandem aldol reaction/siloxy-Cope rearrangement. In all of these cases, the products are generated with very high diastereocontrol. With a chiral dirhodium tetracarboxylate catalyst such as Rh2(S-DOSP)4 or Rh2(S-PTAD)4, researchers can achieve very high levels of asymmetric induction. Applications of the CHCR reaction include the effective enantiodifferentiation of racemic dihydronaphthalenes and the total synthesis of several natural products: (−)-colombiasin A, (−)-elisapterosin B, and (+)-erogorgiaene. By combining the CHCR reaction into a further cascade sequence, we and other researchers have achieved the asymmetric synthesis of 4-substituted indoles, a new class of monoamine reuptake inhibitors.