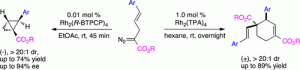Authors: Changming Qin and Huw M. L. Davies
Org. Lett.,
2013, 15 (2), 310–313
The rhodium-catalyzed reaction of 2-diazo-5-arylpent-4-enoates can be controlled by the appropriate choice of catalyst and catalyst loading to form either 2-arylbicyclo[1.1.0]butane carboxylates or cyclohexene derivatives. Both products are produced in a highly diastereoselective manner, with 2-arylbicyclo[1.1.0]butane carboxylates preferentially formed under low catalyst loadings. When the reaction is catalyzed by Rh2(R-BTPCP)4, the 2-arylbicyclo[1.1.0]butane carboxylates are generated with high levels of asymmetric induction (70–94% ee).