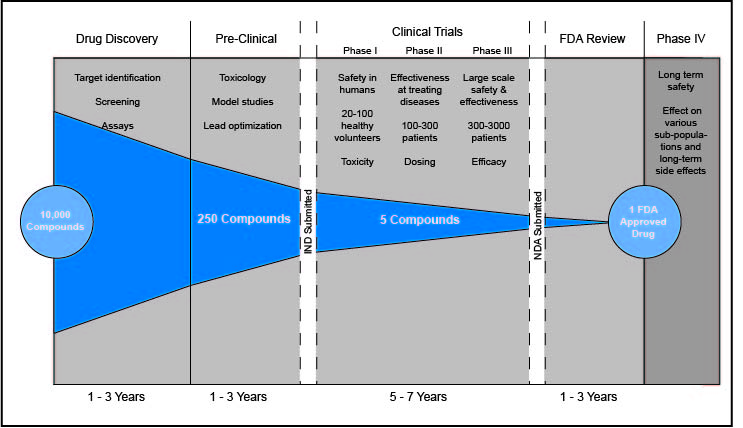
Any time a new drug appears on the market, the final product is a result of years of research and testing. The time from conception to FDA approval for medications takes 12 years on average. An important and time-consuming aspect of this process are clinical trials. By testing a drug on gradually increasing numbers of patients and volunteers, researchers are able verify that it is both safe and effective in treating the disease or condition. Only when the first three steps of this process are complete can the drug be approved and commercially distributed.
Phase 1 is the shortest of the three phases and involves the smallest sample size. This phase is the first time a new drug is tested on humans and establishes the product’s safety. During Phase 1, the drug is given to 20 to 50 volunteers. Generally, this process takes several months. By examining subjects over this period, researchers are able to determine the appropriate dosage, observe side effects, and glean limited information about the drug’s effectiveness. These data are taken into consideration as researchers plan for Phase 2, which approximately 70% of drugs move onto.
Phase 2 gives researchers a clearer picture of a drug’s efficacy and safety. With preliminary safety data available, testing can be expanded to groups of up to several hundred patients. While these samples typically aren’t large enough to fully test a drug’s effectiveness, the results provide further insight into whether it is safe. Phase 2 trials last several months to two years. They are critical in winnowing out unsafe or unsuccessful drugs, with only approximately 33% of drugs proceeding to Phase 3.
The longest and most complex step of clinical trials is Phase 3, which involves 30 to 300 patients and takes 1 to 4 years. Phase 3 answers the most critical question for any drug: whether it provides the beneficial treatment it was designed to. Researchers test this by randomizing the study’s participants. Half of patients receive the experimental drug, while the other half are given a placebo. Studies are usually double-blind, which means neither the participant nor the researchers know who is in which group. Results from the two groups are then compared to determine the effectiveness of the drug. Phase 3 also provides additional safety data by sometimes revealing side effects that went undetected in smaller sample groups. Approximately 25 to 30% of drugs complete this phase and are ready for FDA approval.
Once drugs are FDA approved and commercially available, further testing continues in Phase 4 clinical trials. Although the drug has already been approved, Phase 4 allows for analysis of any long-term effects. Additionally, Phase 4 allows for more organic testing among groups that may not have been studied due to the controlled nature of previous phases, such as those simultaneously taking other drugs. Sometimes, drugs are banned from use by the FDA after harmful side effects emerge during Phase 4.
Clinical trials are a time-consuming, expensive, and selective process. Drugs that make it through and are FDA approved have an average cost of $41,117 per patient just for clinical trials. The federal government has created a database that is home to information about both privately and publicly funded clinical studies around the world. However, clinical trials remain the most comprehensive method available to ensure that every drug marketed in the U.S. is both safe and effective. Doctors and patients can have confidence knowing the drugs they prescribe, and use, have been put to this test and passed.