(1) Catalytic Oxidation of Reduced POM by Dioxygen
(2) Counter-Cation Effect on POMs
Catalytic Oxidation of Reduce POM by Dioxygen
POMs can oxidize a wide variety of substrates, employing O2 to reoxidize the POM catalyst. However, this reoxidation step can be rate-limiting. The reoxidation of POMs by dioxygen can be efficiently catalyzed by copper complexes. Reaction kinetics and mechanisms have been studied to quantitatively describe experimental kinetic curves over a wide variety of conditions.
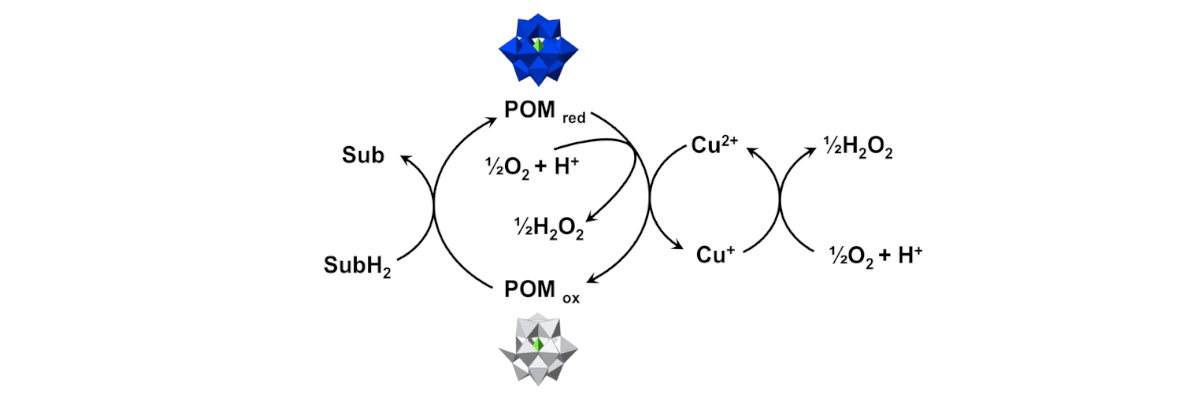
ACS Catal., 2015, 5, 7048.
Counter-Cation Effect on POMs
POM countercations are generally overlooked, as typically their identities are assumed to not impact reactivity; however, we have observed specific K+ cation effects on (a) spectroscopic, (b) kinetic, (c) acid-base and (d) electrochemical properties in the well-established Ru4Si2 molecular WOC.
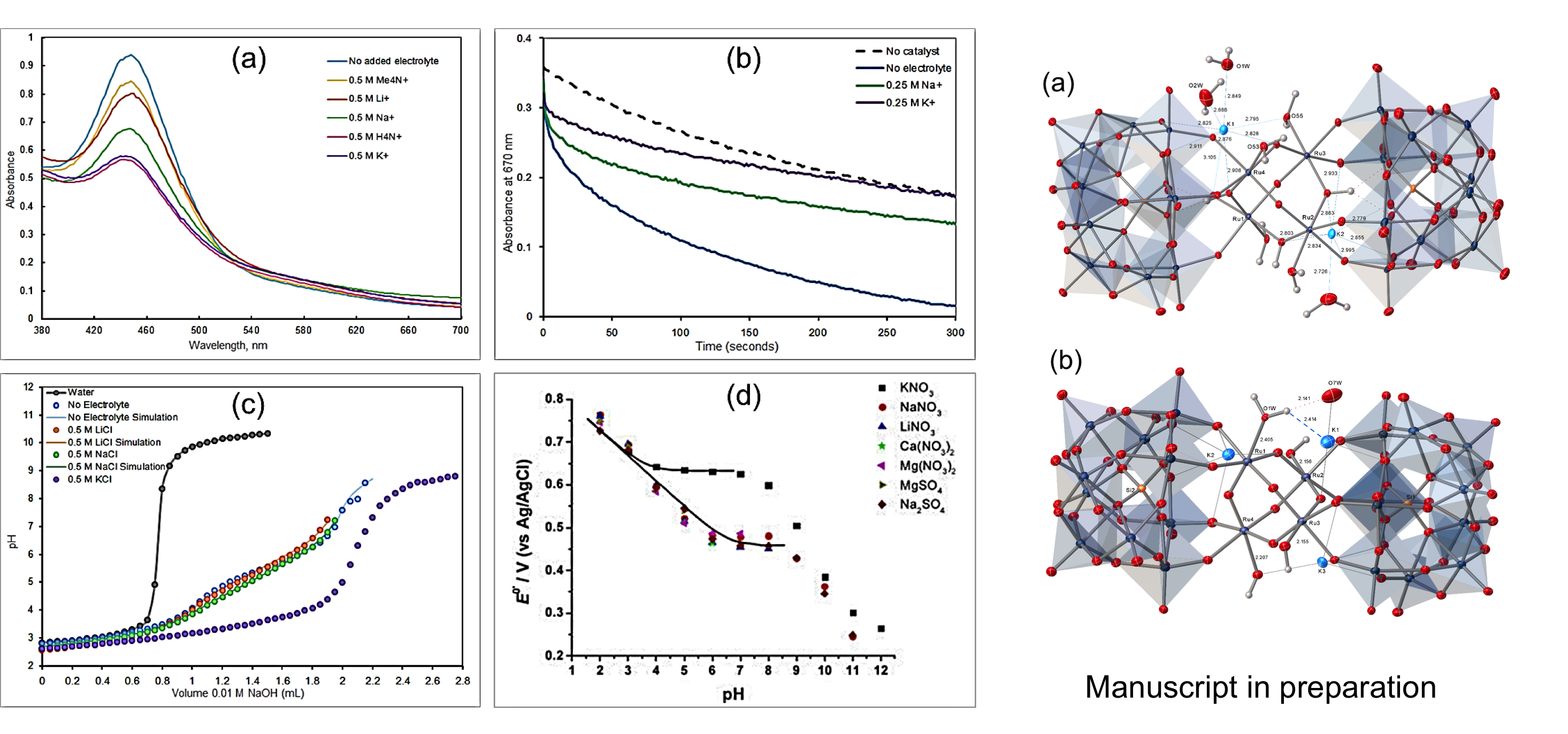
X-ray crystallography has identified specific binding pockets capable of coordinating K+ ions proximally to the catalytic reaction center in reduced states or at elevated pH in Ru4Si2.