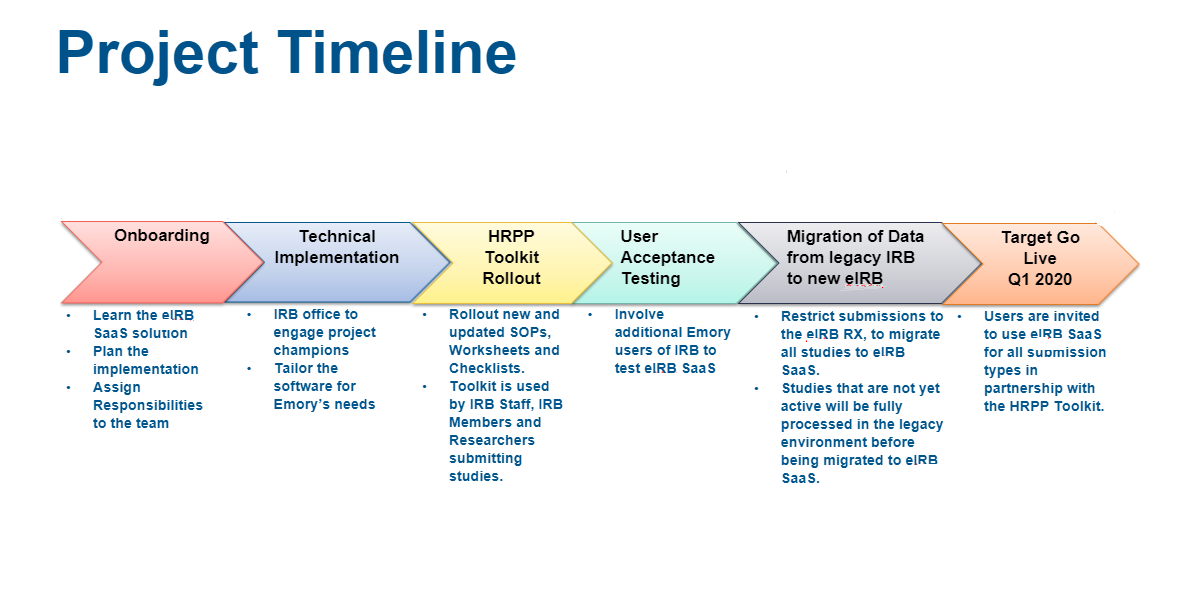
IRB is actively working with our system vendor, Huron, on updating our current electronic system. Currently, we are actively working on updating templates, guidance and other documents that you will need when we launch the new eIRB system, scheduled for Q1 2020. The development process is intensive – so please bear with our staff as we attend many design and training sessions. The end result should be:
- Streamlined submission forms for investigators (many fewer smartform pages!)
- More robust help/guidance along with required protocol templates for investigator-initiated studies
- Shorter forms for IRB Committee reviewers
- Less burden on IRB staff and IT to maintain the system over time
The good news is that the platform, from the same vendor as our current system (and the new eIACUC), will look quite familiar. The launch will require a new submission freeze in our current system, as data is migrated. We will alert you well in advance when this is coming.
The documents expected to change would be as follows:
- Protocol Templates
- Checklist and Worksheets
We will not modify (or they will be minimally modified) our current Policies and Procedures and consent/HIPAA authorization forms. In addition, the IRB is hosting monthly webinars to update the Emory research community about the latest progress in this big project. To find more information about our webinars, go to the IRB webinar page. We have a dedicated page on our website to inform you about our progress. Click on the image below to be directed the progress page or follow this link.