During these unprecedented times, Emory researchers are working hard to understand the coronavirus as quickly as possible through research ranging from vaccine clinical trials to basic science exploration of the virus’s properties. Through hard work and flexibility, many ORA individuals are working hard to facilitate quick coronavirus research and ensure that other research operations continue seamlessly.
Between March 2 and May 12, the IRB had received 103 new study submissions related to COVID. Of these, 56 have already been approved. In addition, 256 COVID-related modifications had been received, e.g. modifications to move to remote study visits. In a more typical year, we receive about 300 modifications per month total.
IRB metrics also indicate how COVID has impacted ongoing research: 49 reportable events had been generated that mention COVID in some capacity. Of these, 40 had been fully reviewed.
Concurrently, other research continues: the IRB had received 425 new studies that did not mention COVID, during that same timeframe (53 of which were local submissions for external IRB studies).
The IRB is prioritizing the review of COVID-related research. The mean (median) turnaround time to approval for COVID-related modifications has been 6 (4) calendar days. For new COVID-related studies, approval has been granted within a mean (median) of 10 (8.5) calendar days, 5.5 days for full-board clinical trials specifically; this is significantly below the IRB’s normal turnaround times for non-COVID studies during this period (19.5(17)). For COVID clinical trials reviewed by an external IRB, the IRB’s local sign-off was completed within 2 calendar days.
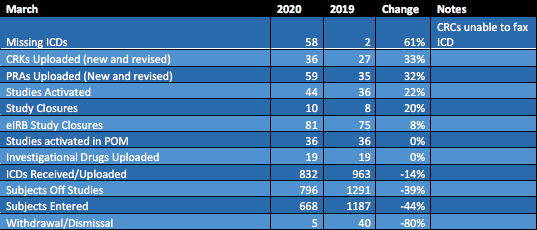
COVID has provided an opportunity to assess changes in ORA workflow due to pandemic-related research. While some campus research has slowed down as a result of the pandemic, COVID-centric research has ramped up in areas ranging from patient-centered vaccine trials to basic science. In response, the ORA’s activities have evolved to accommodate time-sensitive COVID research. Below is a table of OCR metrics in March of 2020 (the first month of shelter-in-place measures and research ramp-down), compared to 2019.
The COVID impact seems to appear on a quarterly basis as well.
While many Emory employees have been working from home, OCR has had representatives on campus. While research has ramped down, many federal sponsors have continued to mail hard copy check payments. Between March 18 and May 1, OCR team members have retrieved and processed 172 checks. This has resulted in $1,381,256.64 in funds posting to PIs’ accounts.