On January 25, 2021, an announcement was released from the EVP for Business and Administration, Christopher Augostini, regarding the restructuring of the University Compliance Office and the establishment of a new Research Compliance Office. The announcement in its entirety, can be found here.
After the retirement of the former leader of the Office of Compliance, Kris West, the EVP for Business and Administration retained an external firm to perform an external review of the office and delivered a formal report to leadership.
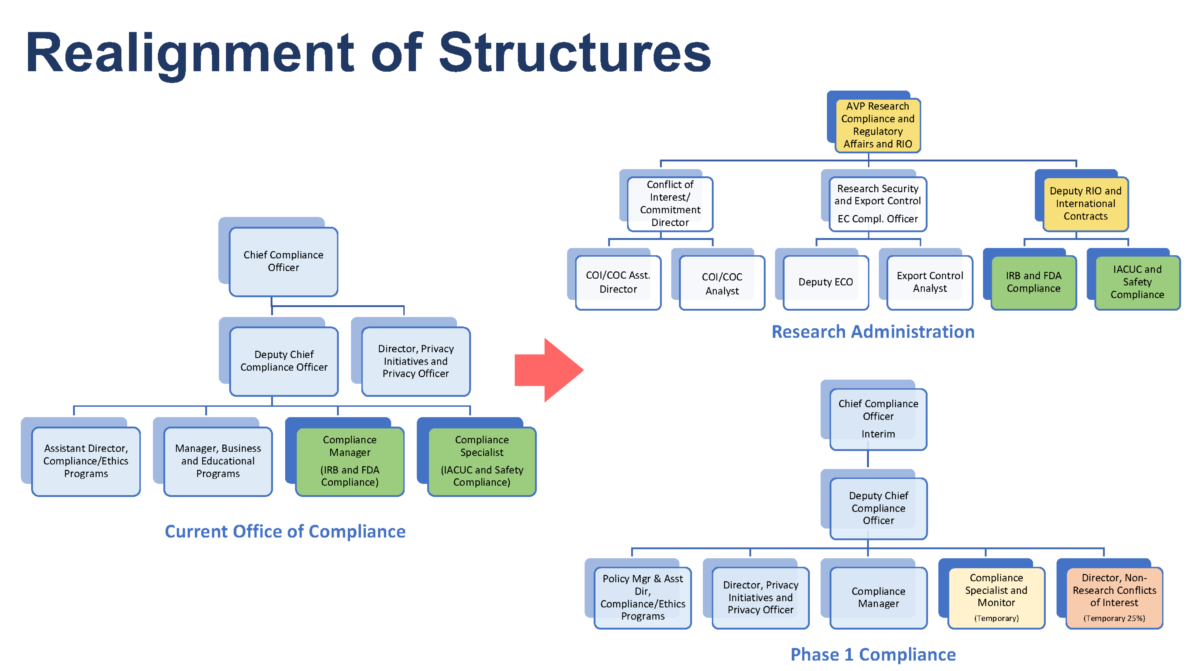
This report illuminated the need for the Office of Compliance to oversee the university’s compliance efforts more holistically, in terms of compliance coordination, oversight and monitoring. Therefore, realigning research-related compliance functions under Emory’s Office of Research Administration.
With this realignment, some of the current central compliance responsibilities, such as management of research integrity investigations and research compliance-related responsibilities will transition to the Office of Research Administration. It is important to note that the Office of Compliance will continue to have accountability for university compliance oversight and will work closely with the Office of Research Administration and Emory Healthcare compliance offices. An ORA overview of the realignment of structures can be seen in the graphic below:
While an executive search is being conducted, as of February 1, 2021, Dr. Kimberly Eck, AVP for Research, has served as Interim AVP for Research Compliance and Regulatory Affairs. In this role, Dr. Eck facilitates the development of the Office of Research Compliance and Regulatory Affairs and provides direct regulatory support and compliance services to the extensive research mission of Emory University.
Some additional functions under the Office of Research Compliance and Regulatory Affairs are:
- Research Misconduct and Non-compliance Investigations
- Research policy and procedure creation and review
- Regulatory non-compliance and sponsor reporting
- DEA and other dangerous drugs oversight
- Contract Negotiations/Terms related to compliance considerations (HIPAA, Data Use, Indemnification, risk assessment of new drugs/devices, etc.)
- New research regulations assessment
- Clinical trial agreements
- Clinical trial patient reimbursement challenges that surface
- Liaising all ORA FOIA requests
- IRB, IACUC, Biosafety, Radiological Safety, Chemical Safety support, accreditation, and compliance
- Regulatory site visits and inspections (e.g. USDA, OSHA complaints, etc.)
- Negotiating conflicts between state and federal laws (e.g. HIV testing with minors)
- Liaison with counsel and external counsel (counsel on retainer)
- Liaison with a Contract Research Organization (CRO on retainer for PI initiated multi-site clinical trials)
Some updates as of May 2021:
- The COI Office is preparing to implement a more efficient technology solution to facilitate disclosures in Fall 2021/Spring 2022.
- After an external review, the Export Control Office is partnering with multiple offices to enhance the infrastructure and protections for covered information, equipment, materials, and locations.
Research Compliance is reviewing and updating their standard operating procedures for partnering with academic units related to non-compliance and research misconduct allegations that are received.