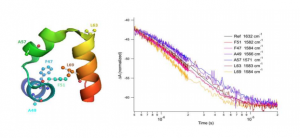
“Time-Resolved Infrared Spectroscopy Reveals the pH-Independence of the First Electron Transfer Step in the [FeFe] Hydrogenase Catalytic Cycle” M. L. K. Sanchez; S. Wiley; E. Reijerse; W. Lubitz; J. A. Birrell; and R. B. Dyer. J. Phys. Chem. Lett. 2022, link

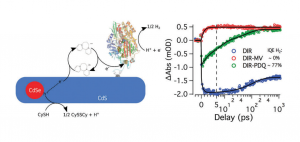
“Efficient, Light-Driven Reduction of CO2 to CO by a Carbon Monoxide Dehydrogenase–CdSe/CdS Nanorod Photosystem” D. W. White; D. Esckilsen; S. K. Lee; S. W. Ragsdale; and R. B. Dyer. J. Phys. Chem. Lett. 2022, link

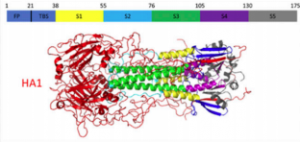
“Stability of HA2 Prefusion Structure and pH-Induced Conformational Changes in the HA2 Domain of H3N2 Hemagglutinin.” M. W. Eller; H. M. H. Siaw; R. B. Dyer. Biochemistry 2021, link

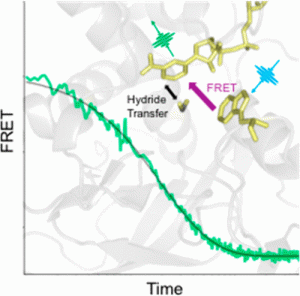
“Acceleration of catalysis in dihydrofolate reductase by transient, site-specific photothermal excitation,” R. Kozlowski; J. Zhao; R.B. Dyer. PNAS, 2021, link



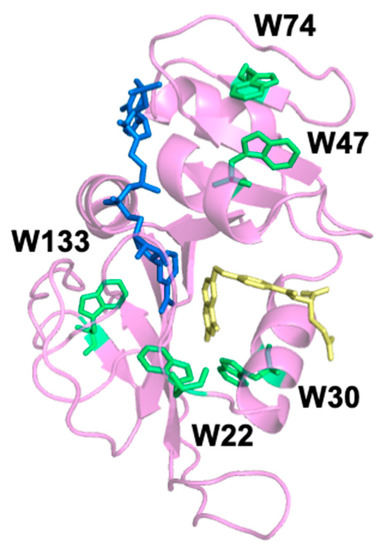
“Site-Specific Tryptophan Labels Reveal Local Microsecond–Millisecond Motions of Dihydrofolate Reductase,” M.B. Vaughn; C. Biren; Q. Li; A. Ragupathi; R.B. Dyer. Molecules, 2020, link
“Metal–ligand cooperativity in the soluble hydrogenase-1 from Pyrococcus furiosus,” G. E. Vansuch; C. Wu; D. K. Haja; S. A. Blair; B. Chica; M. K. Johnson; M.W.W. Adams; R.B. Dyer. Chemical Science, 2020 link
“An Abrupt Change of Configuration of Surface Ligands Affects the H 2 Production Efficiency of Mediator-Based CdS Nanorod/Hydrogenase Assemblies,” W. Yang; G.E. Vansuch; Y. Liu; T. Jin; A. Ge; M.L.K Sanchez; R.B. Dyer; T Lian. 236th ECS Meeting (October 13-17, 2019), link
“Investigating the Kinetic Competency of CrHydA1 [FeFe] Hydrogenase Intermediate States via Time-resolved Infrared Spectroscopy,” M.L.K. Sanchez; C. Sommer; E. Reijerse; J.A. Birrell; W. Lubitz; R.B. Dyer. JACS, 2019 link

“Kinetics of Histidine-Tagged Protein Association to Nickel-Decorated Liposome Surfaces,” G. Raghunath; R.B. Dyer. Langmuir, 2019 , 35, 12550-12561 link

“Active Site Glu165 Activation in Triosephosphate Isomerase and its Deprotonation Kinetics,” H. Deng; R.B. Dyer; R. Callender. J. Phys. Chem. B., 2019 link

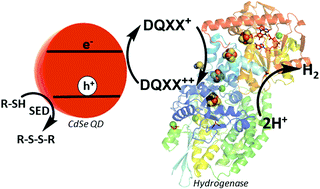
“Optimizing electron transfer from CdSe QDs to hydrogenase for photocatalytic H2 production,” M. Sanchez; Wu, C.; Adams, M.W.W.; R.B. Dyer. ChemComm, 2019 link
“Localized Nanoscale Heating Leads to Ultrafast Hydrogel Volume-Phase Transition,” J. Zhao; H. Su; G. E. Vansuch; Z. Liu; K. Salaita; R.B. Dyer. ACS Nano, 2018 link

“Dynamics of hemagglutinin-mediated membrane fusion,” M.W. Eller, R.B. Dyer. PNAS, 2018 link
“Heterogeneity in the Folding of Villin Headpiece Subdomain HP36,” S. Nagarajan, S. Xiao, D.P. Raleigh, R.B. Dyer. J. Phys. Chem. B, 2018 Just Accepted link
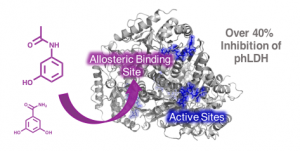
“Small molecule cores demonstrate non-competitive inhibition of lactate dehydrogenase,” B. Andrews, R.B. Dyer Med. Chem. Comm., 2018 Accepted link
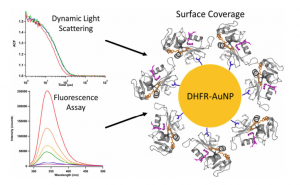
“Characterizing the Surface Coverage of Protein-Gold Nanoparticle Bioconjugates,” R. B. Kozlowski; A. Ragupathi; R.B. Dyer Bioconjugate Chemistry, 2018, 29, 2691–2700. link
“A New Era for Electron Bifurcation,” J. W. Peters, D. N. Beratan, B. Bothner, R. B. Dyer, C. S. Harwood, Z. M. Heiden, R. Hille, A. K. Jones, P. W. King, Y. Lu, C. E. Lubner, S. D. Minteer, D. W. Mulder, S. Raugei, G. J. Schut, L. C. Seefeldt, M. Tokmina-Lukaszewska, O. Zadvornyy, P. Zhang, and M. W. W. Adams, Curr. Opin. Chem. Biol. 2018, 47, 32-38 link

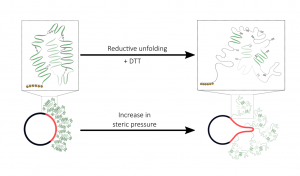
“Peripheral Protein Unfolding Drives Membrane Bending,” Siaw, H.M.H.; Raghunath, G.; Dyer, R.B. Langmuir, 2018, 34, 8400–8407. link
“Light-Responsive Polymer Particles as Force Clamps for the Mechanical Unfolding of Target Molecules,” Su, H.; Liu, Z.; Liu, Y.; Ma, V.P.; Blanchard, A.; Zhao, J.; Dyer, R.B.; Salaita, K. Nano Letters, 2018, 18, 2630-2636. link

Dyer, R. B.; Reddish, M. J.; Callender, R. “Protein Dynamics in Enzymatic Catalysis.” In Catalysis in Chemistry and Biology. Proceedings of the 24th International Solvay Conference on Chemistry; Wüthrich, K., Grubbs, R. H., Eds., 2018; pp 303-308
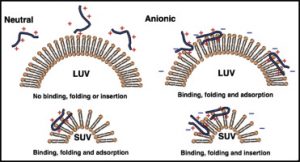
“Binding, folding and insertion of a β-hairpin peptide at a lipid bilayer surface: Influence of electrostatics and lipid tail packing,” Reid, K.A.; Davis, C.M.; Dyer, R.B.; Kindt, J.T. Biochimica et Biophysica Acta- Biomembranes, 2018, 3, 792- 800 link
“Activity-Related Microsecond Dynamics Revealed by Temperature-Jump Förster Resonance Energy Transfer Measurements on Thermophilic Alcohol Dehydrogenase,”Vaughn, M. B.; Zhang, J.; Spiro, T. G.; Dyer, R. B.; Klinman, J. P., Activity-Related Microsecond Dynamics Revealed by T-Jump FRET Measurements on Thermophilic Alcohol Dehydrogenase. J Am Chem Soc 2018, 140, 900–903. link
“Applications of Photogating and Time Resolved Spectroscopy to Mechanistic Studies of Hydrogenases,” Greene, B.L.; Vansuch, G.E.; Adams, M.W.W.; Dyer, R.B. Accounts of Chemical Research, 2017, 50, 2718–2726.link

“Balancing electron transfer rate and driving force for efficient photocatalytic hydrogen production in CdSe/CdS nanorod-[NiFe] hydrogenase assemblies,” Chica, B.; Wu C.; Liu, Y.; Adams, M.W.W.; Lian T.; Dyer, R. B. Energy & Environmental Science, 2017,10, 2245 – 2255.link
“Parallel folding pathways of Fip35 WW domain explained by infrared spectra and their computer simulation,” Zanetti-Polzi, L.; Davis, C.M.; Gruebele, M.; Dyer, R.B.; Amadei, A.; Diadone, I. FEBS Letters, 2017, 591, 3265–3275.link
“Resolution of Submillisecond Kinetics of Multiple Reaction Pathways for Lactate Dehydrogenase”, M. J. Reddish, R. Callender, and R. B. Dyer, Biophys. J. 2017, 112(9), 1852-1862 link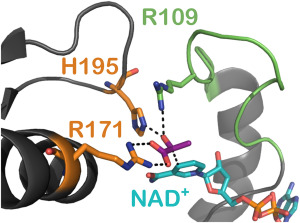
“Proton Transport Mechanism of M2 Proton Channel Studied by Laser-Induced pH Jump”, B. Jeong and R. B. Dyer, J. Am. Chem. Soc. 2017, 139, 6621–6628. PMID28467842
“Pre-Steady-State Kinetics of Catalytic Intermediates of an [FeFe]-Hydrogenase”, B. L. Greene, G. J. Schut, M. W. Adams and R. B. Dyer, ACS Catalysis 2017, 7(3), 2145-2150.Link

“Dual time-resolved temperature-jump fluorescence and infrared spectroscopy for the study of fast protein dynamics”, C. M. Davis, M. J. Reddish and R. B. Dyer, Spectrochim. Acta Mol. Biomol. Spectrosc. 2017, 178, 185-191 Link
“Glutamate Gated Proton-Coupled Electron Transfer Activity of a [NiFe]-Hydrogenase”, B. L. Greene, G. E. Vansuch, C. H. Wu, M. W. Adams and R. B. Dyer, J. Am. Chem. Soc. 2016, 138 (39), 13013–13021 PMID27617712

“Submillisecond Dynamics of Mastoparan X Insertion into Lipid Membranes”, E. E. Schuler, S. Nagarajan, and R. B. Dyer, J. Phys. Chem. Lett. 2016, 7 (17), 3365–3370 PMID27513014

“Proton Inventory and Dynamics in the Nia-S to Nia-C Transition of a [NiFe] Hydrogenase”, B. L. Greene, C. H. Wu, G. E. Vansuch, M. W. Adams and R. B. Dyer, Biochemistry 2016, 55 (12), 1813–1825 PMID26956769

“Ligand Dependent Conformational Dynamics of Dihydrofolate Reductase”, M. J. Reddish, M. B. Vaughn, R. Fu, and R. B. Dyer, Biochemistry. 2016, 55 (10), 1485–1493 PMID26901612

“Synthesis and Catalytic Reactivity of a Dicopper(II) μ-η2:η2-Peroxo Species Supported by 1,4,7-Tri-tert-butyl-1,4,7-triazacyclononane.”, G. J. Karahalis, A. Thangavel, B. Chica, J. Bacsa, R. B. Dyer, C. C. Scarborough, Inorg. Chem. 2016, 55(3), 1102–1107. PMID26789550

“The Role of Electrostatic Interactions in Folding of β-Proteins.”, C. M. Davis, R. B. Dyer, J. Am. Chem. Soc. 2016, 138(4), 1456–1464. PMID26750867

“Sandwich-format 3D printed microfluidic mixers: a flexible platform for multi-probe analysis”, D. Kise, M. J. Reddish, R. B. Dyer, J. Micromech. Microeng.2015, 25, 124002. (http://iopscience.iop.org/0960-1317/25/12/124002/media)
“Proton-Coupled Electron Transfer Dynamics in the Catalytic Mechanism of a [NiFe]-Hydrogenase”, B. L. Greene, C.-H. Wu, P. M. McTernan, M. W. W. Adams, and R. B. Dyer, J. Am. Chem. Soc. 2015, 137, 4558-4566. PMID25790178

“The Pathway of O2 to the Active Site in Heme-Copper Oxidases”, Ó. Einarsdóttir, W. McDonald, C. Funatogawa, W. H. Woodruff and R. B. Dyer, Biochim. Biophys. Acta Bioenergetics 2015, 1847, 109−118. PMID24998308
“Fast Helix Formation in the B Domain of Protein A Revealed by Site-Specific Infrared Probes.”, C. M. Davis, A. K. Cooper, R. B. Dyer, Biochemistry 2015, 54(9), 1758-1766. PMID25706439

“The Dynamical Nature of Enzymatic Catalysis”, R. Callender, R. B. Dyer, Acc. Chem. Res. 2015, 48(2), 407-413. PMID25149276
“Direct Evidence of Catalytic Heterogeneity in Lactate Dehydrogenase by Temperature Jump Infrared Spectroscopy”, M. Reddish, H.-L. Peng, H. Deng, K. S. Panwar, R. Callender, and R. B. Dyer, J. Phys. Chem. B 2014, 118(37), 10854−10862. PMID25149276

“All-inorganic Networks and Tetramer based on Tin(II)-containing Polyoxometalates: Tuning Structural and Spectral Properties with Lone Pairs”, C. Zhao, E. N. Glass, B. Chica, D. G. Musaev, J. M. Sumliner, R. B. Dyer, T. Lian, C. L. Hill, J. Am. Chem. Soc. 2014, 136, 12085−12091. PMID25076405

“CO2 Reduction Catalyzed by Mercaptopteridine on Glassy Carbon”, D. Xiang, D. Magana, R. B. Dyer, J. Am. Chem. Soc. 2014, 136, 14007−14010. PMID25259884

“WW Domain folding complexity revealed by infrared spectroscopy”, C.M. Davis, R. B. Dyer, Biochemistry 2014, 53 (34), 5476-5484.PMID25121968

“Quantum dots encapsulated within phospholipid membranes: phase-dependent structure, photostability, and site-selective functionalization”, W. Zheng, Y. Liu, A. West, E. Schuler, K. Yehl, R. B. Dyer, J. T. Kindt, K. Salaita, J. Am. Chem. Soc. 2014, 136 (5), 1992-1999.PMID24417287

“The Energy Landscape of Lactate Dehydrogenase: Relationship to Catalytic Mechanism”, Peng, H.-L.; Deng, H.; Dyer, R. B.; Callender, R. Biochemistry 2014, 53(11):1849-57 PMID24576110
“The Fate of Excited-State Charge Transfer in Push-Pull Chromophores Depends on the Initial Charge Distribution”, G. Li, D. Magana, C. Kumar and R. B. Dyer, J. Phys. Chem. B 2014.
“Dynamics of an Ultrafast Folding Subdomain in the Context of a Larger Protein Fold”, C. M. Davis and R. B. Dyer, J. Am. Chem. Soc. 2013, 135 (51), 19260-19267. PMID24320936

“Submillisecond mixing in a continuous-flow, microfluidic mixer utilizing mid-infrared hyperspectral imaging detection” D. Kise, D. Magana, M. Reddish, R. B. Dyer, Lab Chip 2014, 14, 584-591. PMID24302515
“Anisotropic Energy Flow and Allosteric Ligand Binding in Albumin”, G. Li, D. Magana, and R. B. Dyer, Nat Commun. 2014, 5, 3100.PMID24445265
“A simple three-dimensional-focusing, continuous-flow mixer for the study of fast protein dynamics”, K. Burke, D. Parul, M. Reddish, R. B. Dyer, Lab Chip 2013, 13, 2912-2921. PMC3733270

“Dynamics of the gel to fluid phase transformation in unilamellar lipid bilayer vesicles,” S. Nagarajan, E. E. Schuler, K. Ma, J. T. Kindt and R. B Dyer, J. Phys. Chem. B 2012, 116 (46), pp 13749–13756. PMC3508262 (http://pubs.acs.org/doi/abs/10.1021/jp309832u)
“Temperature Dependence of Water Interactions with the Amide Carbonyls of α-Helices,” S. H. Brewer, S. Gnanakaran, Y. Tang, D. M. Vu, D. P. Raleigh, R. B. Dyer, Biochemistry 2012, 51 (26), pp 5293–5299. PMC3448027

“Direct evidence of active site reduction and photo-driven catalysis in sensitized hydrogenase assemblies,” R. Dyer, B. L. Greene C. A. Joseph M. J. Maroney J. Am. Chem. Soc. 2012, 134, 11108−11111. PMC3394927

“Raising the speed limit for β-Hairpin formation,” C. M. Davis, S. Xiao, D. P. Raleigh and R. B. Dyer, J. Am. Chem. Soc. 2012, 134 (35), 14476–14482. PMC3443077

“Direct Observation and Control of Ultrafast Photoinduced Twisted Intramolecular Charge Transfer (TICT) in Triphenyl-Methane Dyes” G. Li, D. Magana and R. B. Dyer J. Phys. Chem. B, 2012, 116(41): 12590–12596. PMC3475756
“Catalytic Deoxyribozyme-Modified Nanoparticles for RNAi-Independent Gene Regulation,” K. Yehl, J. P. Joshi, B. L. Greene, R. B. Dyer, R. Nahta, K. Salaita, ACS Nano 2012, 6 (10), 9150–9157. (http://pubs.acs.org/doi/abs/10.1021/nn3034265)

“Early turn formation and chain collapse drive fast folding of the major cold shock protein CspA of E. coli,” D. M. Vu, S. H. Brewer, R. B. Dyer, Biochemistry 2012, 51 (45), pp 9104–9111. (http://pubs.acs.org/doi/abs/10.1021/bi301296y)

“Photoinduced Electron Transfer in Folic Acid Investigated by Ultrafast Infrared Spectroscopy,” G. Li, D. Magana, and R. B. Dyer, J. Phys. Chem. B, 2012, 116, 3467-3475. PMC3311227
“Fast Events in Protein Folding,” R. B. Dyer, D. M. Vu. In: Edward H. Egelman, editor: Comprehensive Biophysics, Vol 3, The Folding of Proteins and Nucleic Acids, Valerie Daggett. Oxford: Academic Press, 2012, pp. 34-42. link
“Time-resolved spectroscopic studies of protein dynamics,” R. Callender; R. B. Dyer, in Encyclopedia of Biophysics, Gordon C. K. Roberts, Editor, 2012.
“Differential Ordering of the Protein Backbone and Side Chains during Protein Folding Revealed by Site-Specific Recombinant Infrared Probes S. Nagarajan, H. Taskent-Sezgin, D. Parul, I. Carrico, D. P. Raleigh, and R. B. Dyer, J. Am. Chem. Soc. 2011, 133, 20335–20340. PMC3241911
Deng, H., D.V. Vu, K. Clinch, R. Desamero, R.B. Dyer, and R. Callender, Conformational heterogeneity within the Michaelis complex of lactate dehydrogenase J. Phys. Chem. B, 2011, 115 (23), pp 7670–7678. link

“Implementation of Time-Resolved Step-Scan Fourier Transform Infrared (FT-IR) Spectroscopy Using a kHz Repetition Rate Pump Laser”, D. Magana, D. Parul, R. B. Dyer, A. P. Shreve, Appl. Spect. 2011, 65, 535-542. PMC3233350
“Formation and Stabilization of Fluorescent Gold Nanoclusters Using Small Molecules” Y. Bao, H.-C. Yeh, C. Zhong, S. A. Ivanov, J. K. Sharma, M. L. Neidig, D. M. Vu, A. P. Shreve, R. B. Dyer, J. H. Werner and J. S. Martinez, J. Phys. Chem. C 2010, 11, 15879-15882. link

“Azidohomoalanine: A Conformationally Sensitive IR Probe of Protein Folding, Protein Structure and Electrostatics,” H. Taskent-Sezgin, J. Chung, P. S. Banerjee, S. Nagarajan, R. B. Dyer, I. Carrico and D. P. Raleigh, Angew. Chem. IE 2010, 49, 7473–7475. (or Angew. Chem. 122, 7635-7637) PMC3233359

2000-2009
“Laser-Induced Temperature Jump Infrared Measurements of RNA Folding,” Dyer, RB; Brauns, EB, Methods in Enzymology: Biophysical, Chemical, and Functional Probes of RNA Structure, Interactions and Folding, Pt B 2009, 469, 353-372. PMC3233360
“Fabrication of fluorescent cellular probes: Hybrid dendrimer/gold nanoclusters,” Zhong, C; Bao, YP; Vu, DM; Dyer, RB; Martinez, JS Matls. Res. Soc. Symp. Proc. 2008, 1007, 265-270.
“On the pathway of forming enzymatically productive ligand-protein complexes in lactate dehydrogenase,” Deng, H., Brewer, S., Vu, D. M., Clinch, K., Callender, R., Dyer, R. B., Biophys. J. 2008, 95, 804-813.
“A simple and economical method for the production of 13C, 18O-labeled Fmoc-amino acids with high levels of enrichment: applications to isotope-edited IR studies of proteins,” J. Marecek, B. Song, S. Brewer, J. Belyea, R. B. Dyer and D. P. Raleigh, Org. Lett. 2007, 9, 4935-4937.
R. B. Dyer and W. H. Woodruff (2007) “Vibrational Spectroscopy,” in Applications of Physical Methods to Inorganic and Bioinorganic Chemistry edited by Robert A. Scott and Charles M. Lukehart. Chichester, UK: John Wiley & Sons, Ltd. pp. 489-512.
“Nanoparticle free synthesis of fluorescent gold nanoclusters at physiological temperature,” J. S. Martinez, Y. Bao, C. Zhong, D. M. Vu, J. P. Temirov, R. B. Dyer, J. Phys. Chem. C 2007, 111, 12194-12198. [1]
“The helix-turn-helix motif as an ultrafast independently folding domain: The pathway of folding of engrailed homeodomain,” T. L. Religa, C. M. Johnson, D. M. Vu, S. H. Brewer, R. B. Dyer, A. R. Fersht, Proc. Natl. Acad. Sci. USA 2007, 104, 9272-9277. [10]
“Residue Specific Resolution of Protein Folding Dynamics Using Isotope-Edited Infrared Temperature Jump Spectroscopy,” S. H. Brewer, B. Song, D. P. Raleigh, and R. B. Dyer, Biochemistry 2007, 46, 3279-3285. [10]
“Ultrafast and Downhill Folding,” R. B. Dyer, Curr. Opin. Struct. Biol. 2007, 17, 38-47. [6]
“Microfluidic Flow-Flash: Method for Investigating Protein Dynamics,” M. W. Toepke, S. H. Brewer, D. M. Vu, K. D. Rector, J. E. Morgan, R. B. Gennis, P. J. A. Kenis, and R. B. Dyer, Anal. Chem. 2007, 79, 122-128. [4]
“Advances in time-resolved approaches to characterize the dynamical nature of enzymatic catalysis,” R. H. Callender, R. B. Dyer, Chem. Rev. 2006, 106, 3031-3042. [11]
“Fourier Transform Hyperspectral Visible Imaging and the Nondestructive Analysis of Potentially Fraudulent Documents,” E. B. Brauns and R. B. Dyer, Appl. Spect. 2006, 60, 833-840. [1]
“A two-dimensional view of the folding energy landscape of cytochrome c,” J. H. Werner, R. Joggerst, R. Keller, R. B. Dyer, P. M. Goodwin, Proc. Natl. Acad. Sci. USA 2006, 103, 11130-11135. [9]
“Time-resolved vibrational spectroscopy detects protein-based intermediates in the photosynthetic oxygen-evolving cycle,” B. A. Barry, I. B. Cooper, A. DeRiso, S. H. Brewer, D. M. Vu, R. B. Dyer, Proc. Natl. Acad. Sci. USA 2006, 103, 7288-7291. [7]
“Nonequilibrium protein folding dynamics: laser-induced pH-jump studies of the helix–coil transition,” T. P. Causgrove and R. B. Dyer, Chem. Phys. 2006, 323, 2-10. [9]
“Effect of modulating unfolded state structure on the folding kinetics of the villin headpiece subdomain,” S. H. Brewer, D. M. Vu, Y. Tang, Y. Li, S. Franzen, D. P. Raleigh and R. B. Dyer, Proc. Natl. Acad. Sci. USA 2005, 102, 16662-16667. [18]
“Studies of Helix Fraying and Solvation using 13C Isotopomers,” R. M. Fesinmeyer, E. S. Peterson, R. B. Dyer and N. H. Andersen, Prot. Sci. 2005, 14, 2324-2332. [7]
“Time-resolved infrared spectroscopy of RNA folding,” E. B. Brauns, R. B. Dyer, Biophys. J. 2005, 89, 3523-3530. [4]
“Hairpin folding dynamics: the cold denatured state is predisposed for rapid refolding, “ R. B. Dyer, S. J. Maness, S. Franzen, R. M. Fesinmeyer, K. A. Olsen, N. H. Andersen, Biochemistry 2005, 44, 10406 – 10415. [16]
“Structural Transformations in the Dynamics of Michaelis Complex Formation in Lactate Dehydrogenase,” S. McClendon, D. M. Vu, K. Clinch, R. H. Callender, R. B. Dyer, Biophys. J. 2005, 89, L7-L9. [7] PMC1289277
“The mechanism of b-hairpin formation,” R. B. Dyer, S. J. Maness, E. S. Peterson, S. Franzen, R. M. Fesinmeyer, N. H. Andersen, Biochemistry 2004, 43, 11560-11566. [37]
“Experimental resolution of early steps in protein folding: testing MD simulations,” D. M. Vu, E. S. Peterson, R. B. Dyer, J. Am. Chem. Soc. 2004, 126, 6546-6547. [13]
“Probing the folding and unfolding dynamics of secondary and tertiary structures in a three-helix bundle protein,” D. M. Vu, J. K. Myers, T. G. Oas, R. B. Dyer Biochemistry, 2004, 43, 3582-3589. [27]
“FTIR Studies of Internal Proton Transfer Reactions Linked to Inter-heme Electron Transfer in Bovine Cytochrome c Oxidase,” B. H. McMahon, M. Fabian, F. Tomson, T. P. Causgrove, J. A. Bailey, F. N. Rein, R. B. Dyer, G. Palmer, R. B. Gennis and W. H. Woodruff, Biochim. Biophys. Acta 2004, 1655, 321-331. [25]
“NMR and Temperature Jump measurements of de novo designed proteins demonstrate rapid folding in the absence of explicit selection for kinetics,” B. Gillespie, D. M. Vu, P. S. Shah, S. A. Marshall, R. B. Dyer, S. L. Mayo, K. W. Plaxco, J. Mol. Biol. 2003, 330, 813-819. [25]
“Nanosecond temperature jump relaxation dynamics of cyclic b-hairpin peptides,” S.J. Maness, S. Franzen, A. C. Gibbs, T. P. Causgrove and R. B. Dyer, Biophys. J. 2003, 84, 3874-3882. [18]
“Primary folding dynamics of sperm whale apomyoglobin: core formation,” M. Gulotta, E. Rogatsky , R. H. Callender and R. B. Dyer, Biophys. J. 2003, 84, 1909-1918. [14]
“Probing protein dynamics using temperature jump relaxation spectroscopy,” Callender, R., Dyer, R.B. Curr. Opin. Struct. Biol. 2002, 12, 628-633. [27]
“Direct Infrared Detection of the Covalently Ring-Linked His-Tyr Structure in the Active Site of the Heme-Copper Oxidases,” F. Tomson, J. A. Bailey, R. B. Gennis, C. J. Unkefer, Z. Li, L. A. Silks, R. A. Martinez, R. J. Donohoe, R. B. Dyer, and W. H. Woodruff, Biochemistry 2002, 41, 14383-14390. [30]
“Towards an Understanding of the Role of Dynamics on Enzymatic Catalysis in Lactate Dehydrogenase,” M. Gulotta, H. Deng, H. Deng, R. B. Dyer, and R. H. Callender, Biochemistry 2002, 41, 3353-3363. [21]
“Time-Resolved Step-Scan Fourier Transform Infrared Spectroscopy of the CO Adducts of Bovine Cytochrome c Oxidase and of Cytochrome bo3 from Escherichia coli,” J. A. Bailey, S. L. Mecklenburg, G. M. MacDonald, A. Katsonouri, A. Puustinen, M. Wikström, R. B. Dyer, R. B. Gennis and W. H. Woodruff, Biochemistry 2002, 41, 2675-2683. [27]
“Photoacoustic cavitation and heat transfer effects in the laser-induced temperature jump in water,” W. O. Wray, T. Aida, R. B. Dyer, Appl. Phys.B. 2002, 74, 57–66. [25]
“Dynamics of the Primary processes of protein folding: Helix nucleation,” J. H. Werner, R. B. Dyer, R. M. Fesinmeyer, N. H. Andersen, J. Phys. Chem. B 2002, 106, 487-494. [47]
ldquo;There is communication between all four Ca2+ binding sites of Calcineurin B,” S. C. Gallagher, Z.-H. Gao, S. Li, R. B. Dyer, J. Trewhella, C. B. Klee, Biochemistry 2001, 40, 12094-12102. [7]
“Human flap endonuclease-1: Conformational change upon binding to the flap DNA substrate and location of Mg2+ binding site,” C.-Y. Kim, M. S. Park and R. B. Dyer, Biochemistry 2001, 40, 3208-3214. [14]
“A Photolysis-Triggered Heme Ligand Switch in H93G Myoglobin,” S. Franzen, J. Bailey, R. B. Dyer, W. H. Woodruff, R. B. Hu, M. R. Thomas, and S. G. Boxer, Biochemistry2001, 40, 5299-5305. [14]
“Core Formation in Apomyoglobin: Probing the Upper Reaches of the Folding Energy Landscape,” R. B. Dyer, R. Gilmanshin, M. Gulotta, R. H. Callender, Biochemistry 2001, 40, 5137-5143. [28]
“Structures of Apomyoglobin’s Various Acid Destabilized Forms,” R. Gilmanshin, M. Gulotta, R. B. Dyer, R. H. Callender, Biochemistry 2001, 40, 5127-5136. [26]
“Resonance Raman Studies of Heme Axial Ligation in H93G Myoglobin,” S. Franzen, S. G. Boxer, R. B. Dyer and W. H. Woodruff, J. Phys. Chem. B 2000, 104, 10359-10367. [18]
“Protein Folding and Unfolding on a Complex Energy Landscape,” D. Thorn-Leeson, F. Gai, H.M. Rodriguez, L. M. Gregoret, R. B. Dyer, Proc. Natl. Acad. Sci. USA 2000, 97, 2527-2532. [66]
“High Spatial Resolution for IR Imaging Using an IR Diode Laser,” J. A. Bailey, R. B. Dyer, D. K Graff, J. R. Schoonover, Appl. Spect. 2000, 54, 159-163. [10]
Pre 2000
“Effect of Hexafluoroisoproponal on the Thermodynamics of Peptide Secondary Structure Formation,” N. H. Andersen, R. B. Dyer, R. M. Fesinmeyer, F. Gai, Z. Liu, J. W. Neidign, H. Tong, J. Am. Chem. Soc. 1999, 121, 9879-9880. [40]
“Dependence of NO Recombination Dynamics on Solution Glycerol Content in Horse Myoglobin,” A. P. Shreve, S. Franzen, M. C. Simpson, and R. B. Dyer, J. Phys. Chem. 1999, 103, 7969-7975. [21]
“Mid-Infrared Spectrum of [Ru(phen)3]2+*,” K. M. Omberg, J. R. Schoonover, S. Bernhard, J. A. Moss, J. A. Treadway, E. M. Kober, R. B. Dyer and T. J. Meyer, Inorg. Chem. 1998, 37, 3505-3508. [11]
“The Unusual Reactivities of Amphitrite Ornata Dehaloperoxidase and Notomastus lobatus Chloroperoxidase Do Not Arise From a Histidine Imidazolate Proximal Heme Iron Ligand,” Franzen, S.; Roach, M. P.; Chen, Y.-P.; Dyer, R. B.; Woodruff, W.H.; Dawson, J.H. J. Am. Chem. Soc. 1998, 120, 4658-4661. [27]
“The Core of Apomyoglobin E-form Folds at the Diffusion Limit,” Gilmanshin, R., Callender, R. H., Dyer, R. B. Nat. Struct. Biol. 1998, 5, 363-365. [33]
“Infrared Studies of Fast Events in Protein Folding,” Dyer, R. B., Feng, G., Woodruff, W. H., Gilmanshin, R., Callender, R. H. Acc. Chem. Res. 1998, 31, 709-716. [121]
“Fast Events in Protein Folding: The Time Evolution of Primary Processes,” R. H. Callender, R. Gilmanshin, R.B. Dyer, and W.H. Woodruff, Ann. Rev. Phys. Chem. 1998, 49, 173-202. [142]
“Time-Resolved Infrared Studies on Two Isomeric Ruthenium(II)/Rhenium(I) Complexes Containing a Nonsymmetric Quaterpyridine Bridging Ligand,” J. R. Schoonover, A. P. Shreve, R. B. Dyer, R. L. Cleary, M. D. Ward, C. A. Bignozzi, Inorg. Chem. 1998, 37,2598-2601. [8]
“Cyanide Binding and Active Site Structure in Heme-Copper Oxidases: Normal Coordinate Analysis of Iron-Cyanide Vibrations of a32+CN- Complexes of Cytochromes ba3 and aa3,” Y. Kim, G. T. Babcock, K.K. Surerus, J. A. Fee, R. B. Dyer, W. H. Woodruff, W. A. Oertling, Biospectroscopy 1998, 4, 1-15. [5]
“Bound Water in the Proton Translocation Mechanism of the Haem-Copper Oxidases,” S. Riistama, G. Hummer, A. Puustinen, R. B. Dyer, W. H. Woodruff, M. Wikstrom, FEBS Lett.1997, 414, 275-280. [115]
“Fourier Transform Infrared Evidence for Connectivity Between CuB and Glutamic Acid 286 in Cytochrome bo3 from E. Coli,” A. Puustinen, J. A. Bailey, R. B. Dyer, S. L. Mecklenburg, M. Wikstrom, W. H. Woodruff Biochemistry 1997,36, 13195-13200. [106]
“Fast Events In Protein Folding: Relaxation Dynamics and Structure of the ‘I’ Form of Apomyoglobin,” R. Gilmanshin, S. Williams, R. H. Callender, W. H. Woodruff and R. B. Dyer Biochemistry 1997, 36, 15006-12. [48]
“Mid-Infrared Spectrum of [Ru(bpy)3]2+*,” K. M. Omberg, J. R. Schoonover, J. A. Treadway, R. M. Leasure, R. B. Dyer, T. J. Meyer J. Am. Chem. Soc. 1997, 119, 7013-7018. [43]
“Structural Heterogeneity of the Various Forms of Apomyoglobin: Implications for Protein Folding,” R. Gilmanshin, R. B. Dyer, and R. H. Callender, Protein Science 1997, 6, 2134-42. [34]
“Fast Events In Protein Folding: Relaxation Dynamics Of Secondary And Tertiary Structure In Native Apomyoglobin,” R. Gilmanshin, S. Williams, R. H. Callender, W. H. Woodruff, and R. B. Dyer, Proc. Natl. Acad. Sci. USA, 1997, 94, 3709-3713. [155]
“Time-Resolved, Step-Scan FTIR Spectroscopy of Excited-States of Transition Metal Complexes”, Schoonover, J. R.; Strouse, G. F.; Omberg, K. M.; Dyer, R. B. Comments on Inorganic Chem. 1996, 18, 165-188. [54]
“Trans Effects in Nitric Oxide Binding to Myoglobin Cavity Mutant H93G”, Decatur, S. M.; Franzen, S.; DePillis, G. D.; Dyer, R. B.; Woodruff, W. H.; Boxer, S. G. Biochemistry1996, 35, 4939-44. [48]
“Picosecond Structural Dynamics of Myoglobin Following Photolysis of Carbon Monoxide”, T. P. Causgrove and R. B. Dyer, J. Phys. Chem. 1996, 100, 3273-7. [33]
“Application of Time-Resolved Vibrational Spectroscopy to the Study of Excited-State Intercomponent Processes in Supramolecular Systems”, C. A. Bignozzi, J. R. Schoonover and R. B. Dyer, invited article, Comm. Inorg. Chem., 1996, 18, 77-100. [12]
“Fast Events in Protein Folding: Helix Melting and Formation in a Small Peptide”, S. Williams, T. P. Causgrove, R. Gilmanshin, K. S. Fang, R. H. Callender, W. H. Woodruff and R. B. Dyer, Biochemistry 1996, 35, 691-7. [490]
“Application of Time-Resolved Step-Scan Fourier Transform Infrared Spectroscopy to Excited-State Electronic Structure in Polypyridyl Complexes of Re(I)”, J. R. Schoonover, G. F. Strouse, R. B. Dyer, W. D. Bates, P. Chen and T. J. Meyer, Inorg. Chem. 1996, 35, 273-4. [80]
“Fast events in protein folding initiated by laser-induced temperature jump and probed by time-resolved infrared spectroscopy”, S. Williams, T. P. Causgrove, R. Gilmanshin, R. B. Dyer, W. H. Woodruff, R. H. Callender, Spectrosc. Biol. Mol., Eur. Conf., 6th (1995), Editor(s): Merlin, Jean Claude; Turrell, Sylvia; Huvenne, Jean Pierre. Publisher: Kluwer, Dordrecht, Neth. 105-6.
“Probing the fast events of protein folding/unfolding”, R. Callender, R. Gilmanshin, T. Causgrove, S. Williams, K. Fang, R. B. Dyer and W. Woodruff in “Frontiers in Interdisciplinary Physics: Biological Physics”, La Jolla International School of Physics, (1995).
“Protein Physics”, R. Callender, R. Gilmanshin, B. Dyer and W. Woodruff, Physics World 1994, 7(8), 41-45. [10]
“Protein response to ligation reactions in myoglobin,” T. P. Causgrove & R. B. Dyer, Springer Proc. Phys. 1994, 74 (Time-Resolved Vibrational Spectroscopy VI), 262-5. [1]
“Spectroscopic characterization of cytochrome ba3, a terminal oxidase from Thermus thermophilus: Comparison of the a3/CuB site to that of bovine cytochrome aa3” Oertling, W. A.; Surerus, K. K.; Einarsdottir, O.; Fee, J. A.; Dyer, R. B.; Woodruff, W. H. Biochemistry 1994, 33, 3128-41. [38]
“Vibrational and Electronic Spectroscopy of Electronically Excited Polychromophoric Ruthenium (II) Complexes,” C. A. Bignozzi, R. Argazzi, C. Chiorboli, F. Scandola, R. B. Dyer, J. R. Schoonover, T. J. Meyer, Inorg. Chem. 1994, 33, 1652-1659. [62]
“Application of Transient Resonance Raman Spectroscopy to the Structure of a Photoinduced Electron-Transfer Intermediate” J. R. Schoonover, Chen, D. Bates, R. B. Dyer, and T. J. Meyer Inorg. Chem. 1994, 33, 793-797. [47]
“Picosecond Infrared Study of the Photodynamics of Carbonmonoxy Cytochrome c Oxidase”, R. B. Dyer, K. A. Peterson, P. O. Stoutland and W. H. Woodruff, Biochemistry1994, 33, 500-507. [42]
“Protein response to ligation reactions in myoglobin,” T. P. Causgrove & R. B. Dyer, Proc. SPIE-Int. Soc. Opt. Eng. 1993, 1890(Biomolecular Spectroscopy III), 64-72.
“Oxidation chemistry of energetic materials in supercritical water”, Harradine, D. M.; Buelow, S.J.; Dell’Orco, P. C.; Dyer, R. B.; Foy, B. R.; Robinson, J. M.; Sanchez, J. A.; Spontarelli, T.; Wander, J.D. Hazard. Waste Hazard. Mater. 1993, 10, 233-46. [40]
“Photodissociation and Recombination of Carbonmonoxy Cytochrome Oxidase: Dynamics from Picoseconds to Kiloseconds”, Einarsdóttir, ´O., Dyer, R.B., Killough, P.M., Fee, J.A., López-Garriga, J.J., Atherton, S.J., Hubig, S.M., Palmer, G., and Woodruff, W. H., Biochemistry 1993, 32, 12013-12024. [94]
“Protein Response to Photodissociation of CO from Carbonmonoxymyoglobin Probed by Time-Resolved Infrared of the Amide I Band”, T. P. Causgrove and R. B. Dyer, Biochemistry 1993, 32, 11985-11991. [24]
“In Situ Raman Spectroscopy of Reactions in Supercritical Water,” D. A. Masten, B. R. Foy, D. M. Harradine and R. B. Dyer, J. Phys. Chem. 1993, 97, 8557-8559. [33]
“Ultrafast Electron Transfer and Coupled Vibrational Dynamics in Cyanide Bridged Mixed-Valence Transition-Metal Dimers,” S. K. Doorn, R. B. Dyer, P. O. Stoutland and W. H. Woodruff, J. Am. Chem. Soc. 1993, 115, 6398-6405. [83]
“Application of Transient Infrared Spectroscopy to intramolecular Energy Transfer in [(phen)(CO)3ReI(NC)RuII(CN)(bpy)2]+”, J. R. Schoonover, K. C. Gordon, R. Argazzi, C. A. Bignozzi, R. B. Dyer, and T.J. Meyer, J. Am. Chem. Soc. 1993,115, 10996-7. [73]
“IR Thermal Imaging of a Monkey’s Head: Local Temperature Changes in Response to Somatosensory Stimulation”, J. S. George, J. D. Lewine, A. S. Goggin, R. B. Dyer and E. R. Flynn, Adv. Exp. Med. Biol. 1993, 333, 125-136. [5]
“The application of time-resolved step-scan FT-IR to the photodynamics of transition metal complexes and heme proteins”, Palmer, R. A.; Plunkett, S. E.; Dyer, R. B.; Schoonover, J. R.; Meyer, T. J.; Chao, J. L. Proc. SPIE-Int. Soc. Opt. Eng. 1993, 2089, 488-9. [1]
“Spectroscopy, Dynamics and Function of Cytochrome Oxidase”, W. H. Woodruff, R. B. Dyer and O. Einarsdottir, in “Biomolecular Spectroscopy” (Advances in Spectroscopy, v21, Part B) R. J. H. Clark and R. E. Hester, eds., London, John Wiley & Sons Ltd., pp. 189-233 (1993).
“Picosecond infrared study of carbonmonoxy cytochrome c oxidase: ligand transfer dynamics and binding orientations,” K. A. Peterson, P. O. Stoutland, R. B. Dyer, and W. H. Woodruff, Springer Proc. Phys. 1992, 68 (Time-Resolved Vib. Spectrosc. V), 24-7.
“Resonance Raman studies of Rieske-type proteins,” D. Kuila, J. R. Schoonover, R. B. Dyer, C. J. Batie, D. P. Ballou, J. A. Fee and W. H. Woodruff, Biochim. Biophys. Acta1992, 1140, 175-183. [41]
“Electronic Coupling in Cyano-Bridged Ruthenium Polypyridine Complexes and Role of Electronic Effects on Cyanide Stretching Frequencies”, C. A. Bignozzi, R. Argazzi, J. R. Schoonover, K. Gordon, R. B. Dyer, F. Scandola, Inorg. Chem. 1992, 31, 5260-5267. [138]
“Ultrafast Infrared Spectroscopy”, P. O. Stoutland, R. B. Dyer, W. H. Woodruff, Science1992, 257, 1913-1917. [57]
“Picosecond Infrared Study of Ultrafast Electron Transfer and Vibrational Energy Relaxation in a Mixed-Valent Ruthenium Dimer”, Doorn, S. K.; Stoutland, P. O.; Dyer, R. B.; Woodruff, W. H. J. Am. Chem. Soc. 1992, 114, 3133-4. [60]
“The time-resolved infrared spectroscopy of Rh2(1,3-diisocyanopropane)4(BPh4)2”, Doorn, S. K.; Gordon, K. C.; Dyer, R. B.; Woodruff, W. H. Inorg. Chem. 1992, 31, 2284-5. [15]
“Reaction of cyanide with cytochrome ba3 from Thermus thermophilus: spectroscopic characterization of the Fe(II)a3•CN::CuB(II)•CN complex indicates four N atoms are coordinated to CuB”, Surerus, K. K.; Oertling, W. A.; Fan, C.; Gurbiel, R. J.; Einarsdottir, O.; Antholine, W. E.; Dyer, R. B.; Hoffman, B. M.; Woodruff, W. H.; Fee, J. A. Proc. Natl. Acad. Sci. U. S. A. 1992, 89, 3195-3199. [43]
“Nature and functional implications of the cytochrome a3 transients after photodissociation of carbon monoxide-cytochrome oxidase”, Woodruff, W. H.; Einarsdottir, O.; Dyer, R. B.; Bagley, K. A.; Palmer, G.; Atherton, S. J.; Goldbeck, R. A.; Dawes, T. D.; Kliger, D. S. Proc. Natl. Acad. Sci. U. S. A. 1991, 88(6), 2588-92. [126]
“Ultrafast photoinduced ligand transfer in carbonmonoxy cytochrome c oxidase. Observation by picosecond infrared spectroscopy”, Dyer, R. B.; Peterson, K. A.; Stoutland, P. O.; Woodruff, W. H. J. Am. Chem. Soc. 1991, 113(16), 6276-7. [41]
“Time-resolved infrared studies of the dynamics of ligand binding to cytochrome c oxidase”, Dyer, R. Brian; Peterson, Kristen A.; Stoutland, Page O.; Einarsdottir, Olof; Woodruff, William H. Proc. SPIE-Int. Soc. Opt. Eng. 1991, 1432(Biomol. Spectrosc. 2), 197-204.
“Ultrafast and not-so fast dynamics of cytochrome oxidase: the ligand shuttle and its possible functional significance”, Woodruff, William H.; Dyer, R. Brian; Einarsdottir, Olof; Peterson, K. A.; Stoutland, P. O.; Bagley, K. A.; Palmer, Graham; Schoonover, J. R.; Kliger, David S. Proc. SPIE-Int. Soc. Opt. Eng. 1991, 1432(Biomol. Spectrosc. 2), 205-10.
“Near-infrared-excitation resonance Raman spectra of the primary electron donor in photosynthetic reaction centers from Rhodobacter sphaeroides”, Donohoe, R. J.; Dyer, R. B.; Swanson, B. I.; Violette, C. A.; Frank, H. A.; Bocian, D. F. J. Am. Chem. Soc. 1990, 112(18), 6716-18. [32]
“Spectroscopy and Structure of Quadruply Bonded Complexes Re2X82- (X = F, Cl and Br) and Mo2Cl4(PMe3)4 Under Extreme Pressure”, D. E. Morris, C. D. Tait, R. B. Dyer, J. R. Schoonover, M. D. Hopkins, A. P. Sattelberger and W. H. Woodruff, Inorg. Chem.1990, 29, 3447-52. [4]
“The Red and Near-Infrared Resonance Raman Spectroscopy of Photo-Induced Defects in the Mixed-Valence Linear Chain Complex [PtII(en)2][PtIV(en)2Cl2][ClO4]4”, R. J. Donohoe, R. B. Dyer and B. I. Swanson, Solid State Commun. 1990, 73, 521-5. [23]
“Fourier Transform Infrared and Resonance Raman Characterization of Cytochrome ba3 from Thermus Thermophilus,” ´O. Einarsdóttir, R. B. Dyer, P. M. Killough, J. A. Fee, and W. H. Woodruff, Proc. SPIE-Int. Soc. Opt. Eng. 1989, 1055, 254-62. [1]
“The Orientation of CO in Carbonmonoxy Cytochrome Oxidase and its Transient Photoproducts. Direct Evidence from Time-Resolved Infrared Linear Dichroism”, R. B. Dyer, J. J. López-Garriga, ´O. Einarsdóttir and W. H. Woodruff, J. Am. Chem. Soc. 1989, 111, 8962-3. [16]
“Transient Binding of Photodissociated CO to CuB+ of Eukaryotic Cytochrome Oxidase at Ambient Temperature. Direct Evidence from Time-Resolved Infrared Spectroscopy”, R. B. Dyer, P. M. Killough, ´O. Einarsdóttir, J. J. López-Garriga and W. H. Woodruff, J. Am. Chem. Soc. 1989, 111, 7657-59. [93]
“A Comparison of the Resonance Raman Properties of the Fast and Slow Forms of Cytochrome Oxidase”, J. R. Schoonover, R. B. Dyer, W. H. Woodruff, G. M. Baker, M. Noguchi and G. Palmer, Biochemistry 1988, 27, 5433-40. [23]
“Resonance Raman Spectroscopy of Blue Copper Proteins,” W. H. Woodruff, R. B. Dyer, and J. R. Schoonover, in “Biological Applications of Raman Spectroscopy,” vol. III, T. G. Spiro, Ed., New York, Wiley, 1988, pp. 413-439.
“Circular Dichroism Studies of the Solution Structures of Chiral Pyridine Substituted Crowns and Their Complexes”, R. B. Dyer, R. G. Ghirardelli, R. A. Palmer, B. A. Jones and J. S. Bradshaw, J. Am. Chem. Soc. 1987, 109, 4780-4786. [14]
“Optical Activity (CD and CPL) As a Probe of Ion Pairing and Solution Structure of Macrocycle Complexes”, R. B. Dyer, R. A. Palmer, R. C. Carter, R. G. Ghirardelli and D. H. Metcalf, in “Understanding Molecular Properties”, A. E. Hansen, J. Avery and J. P. Dahl, Eds.; D. Riedel Publishing Co.: Boston, 1986.
“Crown Ether Complexed Ion Pairs: The Solution and Solid State Structures of the (2S,6S)-2,6-dimethyl-1,4,7,10,13,16-hexaoxacyclooctadecane Complex of Potassium Nitrate”, R. B. Dyer, E. M. Holt, R. G. Ghirardelli and R. A. Palmer, Inorg. Chem. 1986, 25, 3184-3188. [16]
“Circular Dichroism Studies of Crown Complexed Ion Pairs: A Comparison of the Alkali and Alkaline Earth Nitrate Complexes of Chiral Crown Ethers”, R. B. Dyer, D. H. Metcalf, R. G. Ghirardelli, R. A. Palmer and E. M. Holt, J. Am. Chem. Soc. 1986, 108, 3621-3629. [32]