Contributed by: Oceana Hopkins, Arooj Khalid, Kevin Lu
The core idea of modern evolutionary theory is that all life is descended from a common ancestor. Though the theory garners much scrutiny and skepticism, it can be explained in part through the simple mechanism of natural selection. Natural selection takes advantage of the variability that exists within the genome. Random mutations that occur in the genome are behind these variations and sometimes change the fitness of an organism. Natural selection dictates that those with higher fitness survive and reproduce; thus, certain traits are selected for within the species. This process explains how gradual change occurs and how complex organisms arise from simplistic ancestors. A common misconception regarding evolution is that life evolved randomly, or by chance. This misconception could arise because of the random nature of mutations that promote variability. Though randomness is an important component to evolutionary theory, natural selection and survival of those who are more biologically fit make sure that the process is non-random.
Known for their characteristic black and white “tuxedos”, penguins are an aquatic, flightless bird found in both warm and cold climates. Because such a large part of their lives are spent in the water, certain species of penguins will only exit the water to shed their feathers or to mate. Spending nearly 75% of their lives in the water foraging for food, penguins have developed very specific traits optimized for swimming. Utilizing their strong forelimbs to propel their large bodies through the water, penguins have undergone very specific evolutionary changes that allow this mechanism to run smoothly. As discussed in Michael Habib’s paper on structural evolution, the strength of the bones in the forelimbs are significantly greater than that of birds who do not exhibit aqua-flying behavior (2009). In conjunction with increased bone density, more muscle mass also developed and aided in keeping the penguins warm in their cold feeding environment.
Closer inspection of the features that aid water-feeding behavior provides more evidence that disputes the misconception that evolution is random. As seen in 2006 by Slack and colleagues, macroevolution within penguins based on their fossils and mitochondrial genes was tracked and recorded. The evidence showed that the penguins’ bodies gradually adapted to the cold feeding environment over many generations. Additionally, researchers Thomas and colleagues observed in 2010 that cold water penguins have a flow of heat along their wings that originates from the brachial artery called the humeral plexus. This vascular countercurrent heat exchanger (CCHE) provides penguins the opportunity to forage in cold water by limiting heat loss through the flippers. Scientists identified this adaptation through fossil evidence and, upon further research, learned it evolved after penguins lost the ability of aerial flight. Researchers have proposed the CCHE evolved to help balance the energy costs of longer foraging times, since the oceans were significantly cooler than penguin body temperature. Ultimately, researchers have concluded that the humeral plexus was instrumental in allowing penguins to be water feeders in subaquatic environments.
To learn more:
Clarke, J. A., D. T. Ksepka, R. Salas-Gismondi, A. J. Altamirano, M. D. Shawkey, L. D’alba, J. Vinther, T. J. Devries, and P. Baby. “Fossil Evidence for Evolution of the Shape and Color of Penguin Feathers.”Science 330 (2010): 954-57. Print
Fordyce, R. E. and Jones, C. M. 1990. The history of penguins, and new fossil penguin material from New Zealand. Pages 419-446 in Davis, L. S. and Darby, J. D. (editors), Penguin biology. Academic Press, San Diego. 467 p.
Habib, Michael. “The Structural Mechanics And Evolution Of Aquaflying Birds.” Biological Journal of the Linnean Society 99 (2009): 687-98. Print.
Stack, Kerryn E., Craig M. Jones, Tatsuro Ando, G. L. Harrison, R. Ewan Fordyce, Ulfur Arnason, and David Penny. “Molecular Biology and Evolution.” Early Penguin Fossils, Plus Mitochondrial Genomes, Calibrate Avian Evolution. Oxford Journals, Mar. 2006. Web. 13 Nov. 2015.
Subramanian S, Beans-Pico´n G, Swaminathan SK, Millar CD, Lambert DM. 2013 Evidence for a recent origin of penguins. Biol Lett 9: 20130748. http://dx.doi.org/10.1098/rsbl.2013.0748.
Thomas, D. B., D. T. Ksepka, and R. E. Fordyce. “Penguin Heat-retention Structures Evolved in a Greenhouse Earth.” Biology Letters 7 (2010): 461-64. Print.








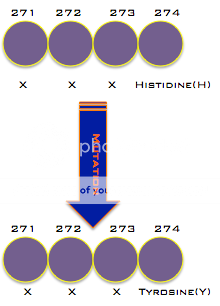 can change genes, often leading to changes in the organism. For Oseltamivir resistance, a point mutation, the change of a single base of DNA, is all that is necessary. A change in the genes leads to a change in the amino acids produced. This change in the amino acids is just one amino acid at the 274th spot in the chain leads to a change in the activity of the virus. This line of reasoning is why the mutation is called H274Y.
can change genes, often leading to changes in the organism. For Oseltamivir resistance, a point mutation, the change of a single base of DNA, is all that is necessary. A change in the genes leads to a change in the amino acids produced. This change in the amino acids is just one amino acid at the 274th spot in the chain leads to a change in the activity of the virus. This line of reasoning is why the mutation is called H274Y.





 In fact, these two rattlesnakes are very closely related, but the populations that live in the mountains have a distinctly different venom than those that live in the desert.
In fact, these two rattlesnakes are very closely related, but the populations that live in the mountains have a distinctly different venom than those that live in the desert.






